Difference between revisions of "Ideal Gas Law"
m (Added category) |
|||
| (8 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | The '''ideal gas law''', is an | + | The '''ideal gas law''', is an equation of state for an ideal gas. It combines three [[gas]] laws ([[Dalton's Law of Partial Pressures|Dalton's Law]], [[Boyle's Law]] and [[Charles' Law]]) into one equation: |
| − | :PV = nRT | + | :<math>PV = nRT</math> |
where | where | ||
| − | *P is the [[pressure]] of gas; | + | *''P'' is the [[pressure]] of gas; |
| − | *V the [[volume]] the gas occupies; | + | *''V'' the [[volume]] the gas occupies; |
| − | *T the absolute [[temperature]] (meaning it must be in [[Kelvin]] or [[Rankine]]); | + | *''T'' the absolute [[temperature]] (meaning it must be in [[Kelvin]] or [[Rankine]]); |
| − | *n is the number of moles of the gas occupying the volume V; | + | *''n'' is the number of [[Mole (chemistry)|moles]] of the gas occupying the volume V; |
| − | *R is the | + | *''R'' is the ideal gas constant. |
| − | The gas constant can be expressed in any number of units, but the most common representations are | + | The ideal gas constant can be expressed in any number of units, but the most common representations are <math>0.0821\,L \cdot atm \cdot mole^{-1} \cdot K^{-1}</math> or <math>8.314\,J \cdot mole^{-1} \cdot K^{-1}</math> |
==Ideal Gas== | ==Ideal Gas== | ||
| − | |||
| − | + | The equation is valid only for an ideal gas, the hypothetically perfect embodiment of a gas in which the particles ([[atom]]s or [[molecule]]s) in the gas are point particles (have no [[volume]]) and experience no intermolecular forces. All collisions between the particles or the particles and the container are perfectly elastic. | |
Since this is just a model, real gases only obey the ideal gas law approximately, not perfectly. Generally, the ideal gas assumption is accurate for unreactive gases at high temperature and/or low pressure. A good rule of thumb is that the assumption can be used above room temperature and below 1 [[atmosphere]] of pressure. | Since this is just a model, real gases only obey the ideal gas law approximately, not perfectly. Generally, the ideal gas assumption is accurate for unreactive gases at high temperature and/or low pressure. A good rule of thumb is that the assumption can be used above room temperature and below 1 [[atmosphere]] of pressure. | ||
| − | [[ | + | ==Density== |
| − | [[ | + | With the molar mass (''M''), the ideal gas law can be used to calculate the [[density]] of a gas, <math>\rho</math>. |
| + | |||
| + | :<math>PM= \rho RT</math> | ||
| + | |||
| + | [[Category:Physics]] | ||
| + | [[Category:Thermodynamics]] | ||
| + | [[Category:Chemistry Laws and Principles]] | ||
Revision as of 18:56, September 8, 2016
The ideal gas law, is an equation of state for an ideal gas. It combines three gas laws (Dalton's Law, Boyle's Law and Charles' Law) into one equation:
where
- P is the pressure of gas;
- V the volume the gas occupies;
- T the absolute temperature (meaning it must be in Kelvin or Rankine);
- n is the number of moles of the gas occupying the volume V;
- R is the ideal gas constant.
The ideal gas constant can be expressed in any number of units, but the most common representations are 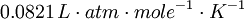 or
or 
Ideal Gas
The equation is valid only for an ideal gas, the hypothetically perfect embodiment of a gas in which the particles (atoms or molecules) in the gas are point particles (have no volume) and experience no intermolecular forces. All collisions between the particles or the particles and the container are perfectly elastic.
Since this is just a model, real gases only obey the ideal gas law approximately, not perfectly. Generally, the ideal gas assumption is accurate for unreactive gases at high temperature and/or low pressure. A good rule of thumb is that the assumption can be used above room temperature and below 1 atmosphere of pressure.
Density
With the molar mass (M), the ideal gas law can be used to calculate the density of a gas,  .
.

