Difference between revisions of "Radiometric dating"
(It is more accurate for shorter time periods (e.g., hundreds of years) during which controlling variables are less likely to change.) |
(restored recent addition of multiple citations as references) |
||
| (32 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '' | + | ''See also [[Counterexamples to an Old Earth]]''. |
| − | + | '''Radiometric dating''' is a method of determining the age of an artifact by assuming that on average decay rates have been constant (see below for the flaws in that assumption) and measuring the amount of radioactive decay that has occurred.<ref>[http://pubs.usgs.gov/gip/geotime/radiometric.html Radiometric Time Scale] USGS</ref> Radiometric dating is mostly used to determine the age of rocks, though a particular form of radiometric dating—called [[Radiocarbon dating]]—can date wood, cloth, skeletons, and other organic material. | |
| − | + | Because radiometric dating fails to satisfy standards of testability and [[falsifiability]], claims based on radiometric dating may fail to qualify under the ''[[Daubert]]'' standard for court-admissible scientific evidence. It is more accurate for shorter time periods (e.g., hundreds of years) during which control variables are less likely to change. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
== Key implausible assumptions == | == Key implausible assumptions == | ||
| − | There are a number of implausible assumptions involved in radiometric dating | + | There are a number of implausible assumptions involved in radiometric dating with respect to long time periods. |
| − | + | ||
=== Initial quantities === | === Initial quantities === | ||
| − | One key assumption is that the initial quantity of the parent element can be determined. With uranium-lead dating, for example, | + | One key assumption is that the initial quantity of the parent element can be determined. With uranium-lead dating, for example, the process assumes the original proportion of uranium in the sample. One assumption that can be made is that all the lead in the sample was once uranium, but if there was lead there to start with, this assumption is not valid, and any date based on that assumption will be incorrect (too old). |
In the case of carbon dating, it is not the initial quantity that is important, but the initial ratio of C<sup>14</sup> to C<sup>12</sup>, but the same principle otherwise applies. | In the case of carbon dating, it is not the initial quantity that is important, but the initial ratio of C<sup>14</sup> to C<sup>12</sup>, but the same principle otherwise applies. | ||
| + | |||
| + | Recognizing this problem, scientists try to focus on rocks that do not contain the decay product originally. For example, in uranium-lead dating, they use rocks containing [[zircon]] (ZrSiO<sub>4</sub>), though it can be used on other materials, such as [[baddeleyite]].<ref>{{Cite journal|doi=10.2113/104.1.13 |title=SHRIMP baddeleyite and zircon ages for an Umkondo dolerite sill, Nyanga Mountains, Eastern Zimbabwe |year=2001 |first=M.T.D. |last=Wingate |journal=South African Journal of Geology |volume=104|issue=1 |pages=13–22}}</ref> Zircon and baddeleyite incorporate uranium atoms into their crystalline structure as substitutes for [[zirconium]], but strongly reject lead. Zincon has a very high closure temperature, is very chemically inert, and is resistant to mechanical weathering. For these reasons, if a rock strata contains zircon, running a uranium-lead test on a zircon sample will produce a radiometric dating result that is less dependent on the initial quantity problem. | ||
=== Rate of decay === | === Rate of decay === | ||
| − | Another assumption is that the rate of decay is constant. | + | Another assumption is that the rate of decay is constant over long periods of time. |
| − | There is no reason to expect that the rate of decay of a radioactive material is largely constant,<ref>At least one example of a change in the rate has been observed in laboratory experiments. See Walker, 2000.</ref> and it was almost certainly not constant near the creation or beginning of the universe. | + | There is no reason to expect that the rate of decay of a radioactive material is largely constant,<ref>At least one example of a change in the rate has been observed in laboratory experiments. See Walker, 2000.</ref> and it was almost certainly not constant near the creation or beginning of the universe.<ref>Multiple references: |
| − | The process of decay is as follows. Atoms consist of a heavy central core called the [[nucleus]] surrounded by clouds of lightweight particles (electrons), called [[electron shell]]s. The energy locked in the nucleus is enormous, but cannot be released easily. The phenomenon we know as heat is simply the jiggling around of atoms and their components, so in principle a high enough temperature could cause the components of the core to break out. However, the temperature required to do this is in in the millions of degrees, so this cannot be achieved by any natural process that we know about. The second way that a nucleus could be disrupted is by particles striking it. However, the nucleus has a strong positive charge and the electron shells have a strong negative charge. Any incoming negative charge would be deflected by the electron shell and any positive charge that penetrated the electron shells would be deflected by the positive charge of the nucleus itself. | + | *Sibley, Andrew (August 2013). [https://creation.com/radioactive-decay-rates-and-solar-activity Variable radioactive decay rates and the changes in solar activity]. ''Creation Ministries International'' (from the ''Journal of Creation 27(2):3–4''). Retrieved January 4, 2018. |
| + | *Wieland, Carl. [https://creation.com/rate-group-reveals-exciting-breakthroughs RATE group reveals exciting breakthroughs!]. ''''Creation Ministries International''. Retrieved January 4, 2018. | ||
| + | *Walker, Tas (April 2000). [https://creation.com/radioactive-decay-rate-depends-on-chemical-environment Radioactive decay rate depends on chemical environment]. ''Creation Ministries International'' (from the ''Journal of Creation 14(1):4–5''). Retrieved January 4, 2018. | ||
| + | *Woodmorappe, John (August 2001). [https://creation.com/billion-fold-acceleration-of-radioactivity-demonstrated-in-laboratory Billion-fold acceleration of radioactivity demonstrated in laboratory]. ''Creation Ministries International'' (from the ''Journal of Creation 15(2):4–6''). Retrieved January 4, 2018. | ||
| + | *Thomas, Brian (August 5, 2009). [http://www.icr.org/article/4816 Radioactive Decay Rates Not Stable]. ''Institute for Creation Research''. Retrieved January 4, 2018. | ||
| + | *Snelling, Andrew A. (October 1, 2009). [https://answersingenesis.org/geology/radiometric-dating/radiometric-dating-problems-with-the-assumptions/ Radiometric Dating: Problems with the Assumptions]. ''Answers in Genesis''. Retrieved January 4, 2018. | ||
| + | *Knapp, Alex (May 3, 2011). [https://www.forbes.com/sites/alexknapp/2011/05/03/radioactive-decay-rates-may-not-be-constant-after-all/#4634f095147f Radioactive Decay Rates May Not Be Constant After All]. ''Forbes''. Retrieved January 4, 2018.</ref> | ||
| + | |||
| + | As early as of 1673, John Ray, an English naturalist, reckoned with alternative that ''"im the primitive times and soon after the Creation the earth suffered far more concussions and mutations in its superficial part than afterward"''.<ref name="Rappaport1997">{{cite book |title=When Geologists Where Historians, 1665-1750 |author=Rhoda Rappaport |publisher=Cornell University Press |place=Ithaca and London |year=1997 |pages=194 |isbn=978-0801-433863 |url=http://books.google.com/books?id=30U3W2kI7foC |quote=}}</ref> The process of decay is as follows. Atoms consist of a heavy central core called the [[nucleus]] surrounded by clouds of lightweight particles (electrons), called [[electron shell]]s. The energy locked in the nucleus is enormous, but cannot be released easily. The phenomenon we know as heat is simply the jiggling around of atoms and their components, so in principle a high enough temperature could cause the components of the core to break out. However, the temperature required to do this is in in the millions of degrees, so this cannot be achieved by any natural process that we know about. The second way that a nucleus could be disrupted is by particles striking it. However, the nucleus has a strong positive charge and the electron shells have a strong negative charge. Any incoming negative charge would be deflected by the electron shell and any positive charge that penetrated the electron shells would be deflected by the positive charge of the nucleus itself. | ||
| + | |||
| + | The decay process is as follows. | ||
| + | |||
| + | Particles consist of various subtypes. Those that can decay are [[meson]]s and [[baryon]]s, which include [[proton]]s and [[neutron]]s; although decays can involve other particles such as [[photon]]s, [[electron]]s, [[positron]]s, and [[neutrino]]s. "Decay" simply refers to a meson or baryon becoming another type of particle, as the number of a certain type of particle goes down or <i>decays</i> as they are converted. This can happen due to one of three forces or "interactions": strong, electromagnetic, and weak, in order of decreasing strength. Historically, these are also known as alpha, gamma, and beta decays, respectively. | ||
| + | |||
| + | "Atomic decays" are due to proton or neutron decays: either weakly, incrementing up or down the table of elements; or strongly, often splitting into smaller elements, one of which is often [[helium]]. For example, a neutron-deficient nucleus may <i>decay weakly</i> by converting a proton in a neutron (to conserve its positive electric charge, it ejects a positron, as well as a neutrino to conserve the quantum [[lepton number]]); thus the hypothetical atom loses a proton and increments <i>down</i> the table by one element. | ||
| + | |||
| + | A complex set of rules describes the details of particle decays: historically, the finding of which as been a major objective of particle physics. Most are determined experimentally by institutions such as [[CERN]] with the [[Large Hadron Collider]]. Decays are very random, but for different elements are observed to conform to statistically averaged different lifetimes. If you had an ensemble of identical particles, the probability of finding a given one of them still as they were - with no decay - after some time is given by the mathematical expression | ||
| + | |||
| + | ::<math>P(t) = e^{-t/(\gamma \tau)} \,</math> | ||
| + | |||
| + | :where | ||
| + | |||
| + | ::<math>\tau</math> is the mean lifetime of the particle (when at rest), proportional to its half-life, and | ||
| + | ::<math>\gamma = \frac{1}{\sqrt{1-v^2/c^2}}</math> is the relativistic [[Lorentz factor]] of the particle. | ||
| + | |||
| + | This governs what is known as the "decay rate." The rate is unique to different particles and so to different atomic elements. This makes different elements useful for different time scales of dating; an element with too short an average lifetime will have too few particles left to reveal much one way or another of potentially longer time scales. Hence, elements such as potassium, which has an average lifetime of nearly 2 billion years before decaying into argon, are useful for very long time scales, with geological applications such as dating ancient lava flows or Martian rocks. Carbon, on the other hand, with a shorter mean lifetime of over 8000 years, is more useful for dating human artifacts. | ||
| + | Atoms themselves consist of a heavy central core called the [[nucleus]] surrounded by arrangements of [[electron shell]]s, wherein there are different probabilities of precisely locating a certain number of electrons (depending on the element). The energy locked in the nucleus is enormous, but cannot be released easily. One way that a nucleus could be disrupted is by particles striking it. However, the nucleus has a strong positive charge and the electron shells have a strong negative charge. Any incoming negative charge would be deflected by the electron shell and any positive charge that penetrated the electron shells would be deflected by the positive charge of the nucleus itself. This interpretation unfortunately fails to consider observed energetic interactions, including that of the strong force, which is stronger the electromagnetic force. | ||
=== Outside influences === | === Outside influences === | ||
| Line 61: | Line 69: | ||
== Acceptance and reliability == | == Acceptance and reliability == | ||
| − | Although radiometric dating methods are widely quoted by [[ | + | Although radiometric dating methods are widely quoted by [[scientists]], they are inappropriate for aging the entire universe due to likely variations in decay rates. Scientists insist that Earth is 4.6 billion years old<ref>hypertextbook.com/facts/1997/MollyMoran.shtml</ref> while the Bible (the infallible word of God<ref>http://www.believers.org/believe/bel191.htm</ref>) suggests that the world to be around 6-10 thousand years old.<ref>http://www.independencebaptist.org/6,000%20year%20old%20earth/6,000_year_old_earth.htm</ref> Because [[Atheism|atheists]] commonly hold the positions of [[Naturalism (philosophy)|naturalism]] and [[uniformitarianism]], many atheists are particularly vocal in claiming that the earth is 4.6 billion years old. |
{{QuoteBox|C14 dating was being discussed at a symposium on the prehistory of the Nile Valley. A famous American colleague, Professor Brew, briefly summarized a common attitude among archaeologists towards it, as follows:<br />"If a C14 date supports our theories, we put it in the main text. If it does not entirely contradict them, we put it in a footnote. And if it is completely 'out of date', we just drop it."<br />Few archaeologists who have concerned themselves with absolute chronology are innocent of having sometimes applied this method...<ref>T. Säve-Söderbergh and I. U. Olsson, ‘C14 dating and Egyptian chronology’, in ''Radiocarbon Variations and Absolute Chronology'', Proceedings of the Twelfth Nobel Symposium, Ingrid U. Olsson (editor), Almqvist & Wiksell, Stockholm, and John Wiley & Sons, Inc., New York, p. 35, 1970. Quoted in Lamb, 2007.</ref>}} | {{QuoteBox|C14 dating was being discussed at a symposium on the prehistory of the Nile Valley. A famous American colleague, Professor Brew, briefly summarized a common attitude among archaeologists towards it, as follows:<br />"If a C14 date supports our theories, we put it in the main text. If it does not entirely contradict them, we put it in a footnote. And if it is completely 'out of date', we just drop it."<br />Few archaeologists who have concerned themselves with absolute chronology are innocent of having sometimes applied this method...<ref>T. Säve-Söderbergh and I. U. Olsson, ‘C14 dating and Egyptian chronology’, in ''Radiocarbon Variations and Absolute Chronology'', Proceedings of the Twelfth Nobel Symposium, Ingrid U. Olsson (editor), Almqvist & Wiksell, Stockholm, and John Wiley & Sons, Inc., New York, p. 35, 1970. Quoted in Lamb, 2007.</ref>}} | ||
| Line 83: | Line 91: | ||
#[[Uranium-thorium]] | #[[Uranium-thorium]] | ||
#[[Rubidium-strontium dating]] | #[[Rubidium-strontium dating]] | ||
| + | |||
| + | == Explanation by Analogy == | ||
| + | |||
| + | No method exists for ''measuring'' [[time]], except by measuring it as it is passing. Therefore, the age of an artifact must be ''calculated''. | ||
| + | |||
| + | The basic principle in any dating method is to find a process that is occurring at a measurable rate and which is causing a change, measure the rate of that process, work out what state the artifact was in at the beginning of the process, observe what state it is in now, and to calculate how long the process at the measured rate would need to occur to effect that change. | ||
| + | |||
| + | For example, to work out how long a [[candle]] has been burning, the following steps would be needed: | ||
| + | # Measure how long it takes the candle to burn down a given amount. | ||
| + | # Find out how long the candle was when it started burning. | ||
| + | # Measure how long the candle is now. | ||
| + | # Calculate the difference between the two lengths. | ||
| + | # Calculate how long it would need to burn in order to burn that length. | ||
| + | |||
| + | For most radiometric dating methods, one radioactive element changes by a process of nuclear decay into another element (often through a number of intermediate steps). For example, [[uranium]] will eventually decay into [[lead]]. So to measure how old a specimen containing some uranium and some lead is, the following steps are required: | ||
| + | # Measure the decay rate of uranium. | ||
| + | # Find out how much uranium was in the specimen to start with (this might be done by assuming that all the lead was originally uranium). | ||
| + | # Find out how much uranium is in the specimen now. | ||
| + | # Calculate how much uranium has turned into lead. | ||
| + | # Calculate how long it would take that much uranium to turn into lead, given the measured rate. Calculations involve the well-established function for exponential decay: <math>F</math><sub><math>t</math></sub><math>=e</math><sup><math>-kt</math></sup>. | ||
| + | |||
| + | |||
== Bibliography == | == Bibliography == | ||
| − | * Lamb, Andrew, [http://creationontheweb.com/content/view/5026 Carbon dating into the future] | + | * Lamb, Andrew, [http://creationontheweb.com/content/view/5026 Carbon dating into the future] 24 March 2007 (Creation Ministries International). |
* Faure, G. and Mensing, T., Isotopes: Principles and Applications, Wiley & Sons, 2005. | * Faure, G. and Mensing, T., Isotopes: Principles and Applications, Wiley & Sons, 2005. | ||
* Walker, M., Quarternary Dating Methods, Wiley & Sons, 2005. | * Walker, M., Quarternary Dating Methods, Wiley & Sons, 2005. | ||
| Line 93: | Line 123: | ||
==Notes and references== | ==Notes and references== | ||
{{reflist|2}} | {{reflist|2}} | ||
| + | |||
| + | ==See also== | ||
| + | *[[Literalist Bible chronology]] ''cites examples of radiometric dating results of artifacts which support a literalist dating of Biblical events such as the Exodus in'' 1577 B.C. | ||
[[Category:Earth Sciences]] | [[Category:Earth Sciences]] | ||
Revision as of 05:34, January 12, 2018
See also Counterexamples to an Old Earth.
Radiometric dating is a method of determining the age of an artifact by assuming that on average decay rates have been constant (see below for the flaws in that assumption) and measuring the amount of radioactive decay that has occurred.[1] Radiometric dating is mostly used to determine the age of rocks, though a particular form of radiometric dating—called Radiocarbon dating—can date wood, cloth, skeletons, and other organic material.
Because radiometric dating fails to satisfy standards of testability and falsifiability, claims based on radiometric dating may fail to qualify under the Daubert standard for court-admissible scientific evidence. It is more accurate for shorter time periods (e.g., hundreds of years) during which control variables are less likely to change.
Contents
Key implausible assumptions
There are a number of implausible assumptions involved in radiometric dating with respect to long time periods.
Initial quantities
One key assumption is that the initial quantity of the parent element can be determined. With uranium-lead dating, for example, the process assumes the original proportion of uranium in the sample. One assumption that can be made is that all the lead in the sample was once uranium, but if there was lead there to start with, this assumption is not valid, and any date based on that assumption will be incorrect (too old).
In the case of carbon dating, it is not the initial quantity that is important, but the initial ratio of C14 to C12, but the same principle otherwise applies.
Recognizing this problem, scientists try to focus on rocks that do not contain the decay product originally. For example, in uranium-lead dating, they use rocks containing zircon (ZrSiO4), though it can be used on other materials, such as baddeleyite.[2] Zircon and baddeleyite incorporate uranium atoms into their crystalline structure as substitutes for zirconium, but strongly reject lead. Zincon has a very high closure temperature, is very chemically inert, and is resistant to mechanical weathering. For these reasons, if a rock strata contains zircon, running a uranium-lead test on a zircon sample will produce a radiometric dating result that is less dependent on the initial quantity problem.
Rate of decay
Another assumption is that the rate of decay is constant over long periods of time. There is no reason to expect that the rate of decay of a radioactive material is largely constant,[3] and it was almost certainly not constant near the creation or beginning of the universe.[4]
As early as of 1673, John Ray, an English naturalist, reckoned with alternative that "im the primitive times and soon after the Creation the earth suffered far more concussions and mutations in its superficial part than afterward".[5] The process of decay is as follows. Atoms consist of a heavy central core called the nucleus surrounded by clouds of lightweight particles (electrons), called electron shells. The energy locked in the nucleus is enormous, but cannot be released easily. The phenomenon we know as heat is simply the jiggling around of atoms and their components, so in principle a high enough temperature could cause the components of the core to break out. However, the temperature required to do this is in in the millions of degrees, so this cannot be achieved by any natural process that we know about. The second way that a nucleus could be disrupted is by particles striking it. However, the nucleus has a strong positive charge and the electron shells have a strong negative charge. Any incoming negative charge would be deflected by the electron shell and any positive charge that penetrated the electron shells would be deflected by the positive charge of the nucleus itself.
The decay process is as follows.
Particles consist of various subtypes. Those that can decay are mesons and baryons, which include protons and neutrons; although decays can involve other particles such as photons, electrons, positrons, and neutrinos. "Decay" simply refers to a meson or baryon becoming another type of particle, as the number of a certain type of particle goes down or decays as they are converted. This can happen due to one of three forces or "interactions": strong, electromagnetic, and weak, in order of decreasing strength. Historically, these are also known as alpha, gamma, and beta decays, respectively.
"Atomic decays" are due to proton or neutron decays: either weakly, incrementing up or down the table of elements; or strongly, often splitting into smaller elements, one of which is often helium. For example, a neutron-deficient nucleus may decay weakly by converting a proton in a neutron (to conserve its positive electric charge, it ejects a positron, as well as a neutrino to conserve the quantum lepton number); thus the hypothetical atom loses a proton and increments down the table by one element.
A complex set of rules describes the details of particle decays: historically, the finding of which as been a major objective of particle physics. Most are determined experimentally by institutions such as CERN with the Large Hadron Collider. Decays are very random, but for different elements are observed to conform to statistically averaged different lifetimes. If you had an ensemble of identical particles, the probability of finding a given one of them still as they were - with no decay - after some time is given by the mathematical expression
- where
 is the mean lifetime of the particle (when at rest), proportional to its half-life, and
is the mean lifetime of the particle (when at rest), proportional to its half-life, and is the relativistic Lorentz factor of the particle.
is the relativistic Lorentz factor of the particle.
This governs what is known as the "decay rate." The rate is unique to different particles and so to different atomic elements. This makes different elements useful for different time scales of dating; an element with too short an average lifetime will have too few particles left to reveal much one way or another of potentially longer time scales. Hence, elements such as potassium, which has an average lifetime of nearly 2 billion years before decaying into argon, are useful for very long time scales, with geological applications such as dating ancient lava flows or Martian rocks. Carbon, on the other hand, with a shorter mean lifetime of over 8000 years, is more useful for dating human artifacts.
Atoms themselves consist of a heavy central core called the nucleus surrounded by arrangements of electron shells, wherein there are different probabilities of precisely locating a certain number of electrons (depending on the element). The energy locked in the nucleus is enormous, but cannot be released easily. One way that a nucleus could be disrupted is by particles striking it. However, the nucleus has a strong positive charge and the electron shells have a strong negative charge. Any incoming negative charge would be deflected by the electron shell and any positive charge that penetrated the electron shells would be deflected by the positive charge of the nucleus itself. This interpretation unfortunately fails to consider observed energetic interactions, including that of the strong force, which is stronger the electromagnetic force.
Outside influences
It is important that the sample not have had any outside influences. One example of this can be found in metamorphic rocks.[6] This does not mean that all rock samples are unreliable, but it is possible to account for a process which throws off the data for metamorphic rocks.
For example, with Uranium-lead dating with the crystallization of magma, this remains a closed system until the uranium decays. As it decays, it disrupts the crystal and allows the lead atom to move. Likewise, heating the rock such as granite forms gneiss or basalt forms schist. This can also disrupt the ratios of lead and uranium in the sample.
Calibration
In order to calibrate radiometric dating methods, the methods need to be checked for accuracy against items with independently-known dates.
Carbon dating, with its much lower maximum theoretical range, is often used for dating items only hundreds and thousands of years old, so can be calibrated in its lower ranges by comparing results with artifacts who's ages are known from historical records.
Scientists have also attempted to extend the calibration range by comparing results to timber which has its age calculated by dendrochronology, but this has also been questioned because carbon dating is used to assist with working out dendrochronological ages.
Otherwise, calibration consists of comparing results with ages determined by other radiometric dating methods.
However, tests of radiometric dating methods have often shown that they do not agree with known ages of rocks that have been seen to form from volcanic eruptions in recent and historic times, and there are also examples of radiometric dating methods not agreeing with each other.
Young earth creationists therefore claim that radiometric dating methods are not reliable and can therefore not be used to disprove Biblical chronology.
Acceptance and reliability
Although radiometric dating methods are widely quoted by scientists, they are inappropriate for aging the entire universe due to likely variations in decay rates. Scientists insist that Earth is 4.6 billion years old[7] while the Bible (the infallible word of God[8]) suggests that the world to be around 6-10 thousand years old.[9] Because atheists commonly hold the positions of naturalism and uniformitarianism, many atheists are particularly vocal in claiming that the earth is 4.6 billion years old.
C14 dating was being discussed at a symposium on the prehistory of the Nile Valley. A famous American colleague, Professor Brew, briefly summarized a common attitude among archaeologists towards it, as follows:
"If a C14 date supports our theories, we put it in the main text. If it does not entirely contradict them, we put it in a footnote. And if it is completely 'out of date', we just drop it."
Few archaeologists who have concerned themselves with absolute chronology are innocent of having sometimes applied this method...[10]
A geological guidebook published by the Queensland government acknowledges that the dates are not absolute, but must be interpreted:
Also, the relative ages [of the radiometric dating results] must always be consistent with the geological evidence. ... if a contradiction occurs, then the cause of the error needs to be established or the radiometric results are unacceptable[11]
One example of scientists not accepting radiometric dates is that of Mungo Man, a human fossil from New South Wales. When originally found, it was dated by radiocarbon dating at around 30,000 years old. This was later revised to 40,000 years. Another scientist later used other methods to derive a date of 62,000 years. The original discoverer, unconvinced by this result, used a different method again, and again came up with a date of 40,000 years.
The fallibility of dating methods is also illustrated by the fact that dating laboratories are known to improve the likelihood of getting a "correct" date by asking for the expected date of the item. For example, the Sample Record Sheet for the University of Waikato Radiocarbon Dating Laboratory asks for the estimated age, the basis for the estimate, and the maximum and minimum acceptable ages.[12]
Some major methods of radiometric dating
There are several major types of radiometric dating in use:[13][14]
- Radiocarbon dating, also called carbon dating
- Potassium-argon dating
- Uranium-lead dating
- Uranium-thorium
- Rubidium-strontium dating
Explanation by Analogy
No method exists for measuring time, except by measuring it as it is passing. Therefore, the age of an artifact must be calculated.
The basic principle in any dating method is to find a process that is occurring at a measurable rate and which is causing a change, measure the rate of that process, work out what state the artifact was in at the beginning of the process, observe what state it is in now, and to calculate how long the process at the measured rate would need to occur to effect that change.
For example, to work out how long a candle has been burning, the following steps would be needed:
- Measure how long it takes the candle to burn down a given amount.
- Find out how long the candle was when it started burning.
- Measure how long the candle is now.
- Calculate the difference between the two lengths.
- Calculate how long it would need to burn in order to burn that length.
For most radiometric dating methods, one radioactive element changes by a process of nuclear decay into another element (often through a number of intermediate steps). For example, uranium will eventually decay into lead. So to measure how old a specimen containing some uranium and some lead is, the following steps are required:
- Measure the decay rate of uranium.
- Find out how much uranium was in the specimen to start with (this might be done by assuming that all the lead was originally uranium).
- Find out how much uranium is in the specimen now.
- Calculate how much uranium has turned into lead.
- Calculate how long it would take that much uranium to turn into lead, given the measured rate. Calculations involve the well-established function for exponential decay:
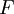


 .
.
Bibliography
- Lamb, Andrew, Carbon dating into the future 24 March 2007 (Creation Ministries International).
- Faure, G. and Mensing, T., Isotopes: Principles and Applications, Wiley & Sons, 2005.
- Walker, M., Quarternary Dating Methods, Wiley & Sons, 2005.
- Walker, Tas., Radioactive decay rate depends on chemical environment, Journal of Creation 14(1):4–5, April 2000.
- Walker, Tas., The way it really is: little-known facts about radiometric dating, Creation 24(4):20–23, September 2002.
Notes and references
- ↑ Radiometric Time Scale USGS
- ↑ Wingate, M.T.D. (2001). "SHRIMP baddeleyite and zircon ages for an Umkondo dolerite sill, Nyanga Mountains, Eastern Zimbabwe". South African Journal of Geology 104 (1): 13–22. doi:10.2113/104.1.13.
- ↑ At least one example of a change in the rate has been observed in laboratory experiments. See Walker, 2000.
- ↑ Multiple references:
- Sibley, Andrew (August 2013). Variable radioactive decay rates and the changes in solar activity. Creation Ministries International (from the Journal of Creation 27(2):3–4). Retrieved January 4, 2018.
- Wieland, Carl. RATE group reveals exciting breakthroughs!. ''Creation Ministries International. Retrieved January 4, 2018.
- Walker, Tas (April 2000). Radioactive decay rate depends on chemical environment. Creation Ministries International (from the Journal of Creation 14(1):4–5). Retrieved January 4, 2018.
- Woodmorappe, John (August 2001). Billion-fold acceleration of radioactivity demonstrated in laboratory. Creation Ministries International (from the Journal of Creation 15(2):4–6). Retrieved January 4, 2018.
- Thomas, Brian (August 5, 2009). Radioactive Decay Rates Not Stable. Institute for Creation Research. Retrieved January 4, 2018.
- Snelling, Andrew A. (October 1, 2009). Radiometric Dating: Problems with the Assumptions. Answers in Genesis. Retrieved January 4, 2018.
- Knapp, Alex (May 3, 2011). Radioactive Decay Rates May Not Be Constant After All. Forbes. Retrieved January 4, 2018.
- ↑ Rhoda Rappaport (1997). When Geologists Where Historians, 1665-1750. Cornell University Press, 194. ISBN 978-0801-433863.
- ↑ Radiometric Dating Course notes for EENS 211 at Tulane University
- ↑ hypertextbook.com/facts/1997/MollyMoran.shtml
- ↑ http://www.believers.org/believe/bel191.htm
- ↑ http://www.independencebaptist.org/6,000%20year%20old%20earth/6,000_year_old_earth.htm
- ↑ T. Säve-Söderbergh and I. U. Olsson, ‘C14 dating and Egyptian chronology’, in Radiocarbon Variations and Absolute Chronology, Proceedings of the Twelfth Nobel Symposium, Ingrid U. Olsson (editor), Almqvist & Wiksell, Stockholm, and John Wiley & Sons, Inc., New York, p. 35, 1970. Quoted in Lamb, 2007.
- ↑ Walker, 2002
- ↑ University of Waikato Radiocarbon Dating Laboratory Sample Record Sheet
- ↑ Walker, 2005
- ↑ Faure and Mensing, 2005
See also
- Literalist Bible chronology cites examples of radiometric dating results of artifacts which support a literalist dating of Biblical events such as the Exodus in 1577 B.C.
