Difference between revisions of "Second Law of Thermodynamics"
(→Probability and statistics: I discovered the "statistical impossibility" page) |
(→Entropy and disorder: improve for clarity and organization) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | The '''Second Law of Thermodynamics''' is a fundamental truth about the tendency towards disorder in the absence of intelligent intervention. This principle correctly predicts that heat will never flow from a cold body to a warmer one, unless forced to do so by a man-made machine. Or, as Isaac Asimov observed, "[t]he universe is constantly getting more disorderly."<ref>Isaac Asimov continued to explain that "[v]iewed that way, we can see the second law all about us. We have to [[work]] to straighten a room, and when left to itself it becomes a mess again very quickly and very easily. Even if we never enter it, it becomes dusty and musty. How difficult to maintain houses, and machinery, and our bodies in perfect working order: how easy to let them deteriorate. In fact, all we have to do is nothing, and everything deteriorates, collapses, breaks down, wears out, all by itself -- and that is what the second law is all about.” Isaac Asimov, Smithsonian Institute Journal, June 1970, p. 6. Put in physics terminology, the Second Law states that the [[entropy]] of an isolated or [[closed system]] never decreases.</ref> | + | The '''Second Law of Thermodynamics''' is a fundamental truth about the tendency towards disorder in the absence of intelligent intervention. This principle correctly predicts that heat will never flow from a cold body to a warmer one, unless forced to do so by a man-made machine. Or, as [[Isaac Asimov]] observed, "[t]he universe is constantly getting more disorderly."<ref>Isaac Asimov continued to explain that "[v]iewed that way, we can see the second law all about us. We have to [[work]] to straighten a room, and when left to itself it becomes a mess again very quickly and very easily. Even if we never enter it, it becomes dusty and musty. How difficult to maintain houses, and machinery, and our bodies in perfect working order: how easy to let them deteriorate. In fact, all we have to do is nothing, and everything deteriorates, collapses, breaks down, wears out, all by itself -- and that is what the second law is all about.” Isaac Asimov, Smithsonian Institute Journal, June 1970, p. 6. Put in physics terminology, the Second Law states that the [[entropy]] of an isolated or [[closed system]] never decreases.</ref> |
The Second Law of Thermodynamics is the result of the intrinsic uncertainty in nature, manifest in [[quantum mechanics]], which is overcome only by intelligent intervention. | The Second Law of Thermodynamics is the result of the intrinsic uncertainty in nature, manifest in [[quantum mechanics]], which is overcome only by intelligent intervention. | ||
| Line 10: | Line 10: | ||
In this context "increasing disorder" means the decline in organization that occurs without intelligent intervention. Imagine your old room at your parent's house. Remember how easy it was to let the room turn into a uniform mess (disorder) and remember how hard it was to clean it up until it fit a specific, non-uniform design (order). Not cleaning up would always result in an increase of entropy in your room! | In this context "increasing disorder" means the decline in organization that occurs without intelligent intervention. Imagine your old room at your parent's house. Remember how easy it was to let the room turn into a uniform mess (disorder) and remember how hard it was to clean it up until it fit a specific, non-uniform design (order). Not cleaning up would always result in an increase of entropy in your room! | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
The flow of energy (by heat exchange) to places with lower concentrations is called the "heat flow." | The flow of energy (by heat exchange) to places with lower concentrations is called the "heat flow." | ||
| − | The often heard argument that this law disproves an eternal universe is true, | + | The often-heard argument that this law disproves an eternal universe is true, because in that case maximum entropy would have been reached already. A counterargument to this would be to suggest that the universe is still in the process of approaching maximum entropy. |
| − | There are many different ways if stating the second law of thermodynamics. An alternative statement of the law is that heat will tend not to flow from a cold body to a warmer one without intelligent | + | There are many different ways if stating the second law of thermodynamics. An alternative statement of the law is that heat will tend not to flow from a cold body to a warmer one without intelligent intervention, or work, being done, as in the case of a [[refrigerator]]. Other statements include that it is impossible for an [[engine]] to convert [[heat]] perfectly (I.e. at 100% efficiency) into [[work]]. These statements are qualitative and stating the second law in terms of [[entropy]] makes the law quantitative. |
This law makes it impossible to build a [[perpetual motion machine]] - the increase in entropy inevitably derails the system even if its [[energy]] remains constant (as described by the [[Conservation of Energy|first law of thermodynamics]]). | This law makes it impossible to build a [[perpetual motion machine]] - the increase in entropy inevitably derails the system even if its [[energy]] remains constant (as described by the [[Conservation of Energy|first law of thermodynamics]]). | ||
| Line 30: | Line 24: | ||
where <math> \frac{dS_{universe}}{dt}</math> is the rate of change of [[entropy]] of the [[universe]] with respect to time. | where <math> \frac{dS_{universe}}{dt}</math> is the rate of change of [[entropy]] of the [[universe]] with respect to time. | ||
| + | |||
| + | Another way of viewing the second law is in terms of probability. In nature there is no one to clean up the universe, only chances. The chance of something becoming orderly is essentially zero, which it is a certainty that things will become more disorderly. | ||
| + | |||
| + | On a universal scale a tidy room would be a universe which has pockets of above average concentrations of energy (if you - incorrectly - assume relativity [[E=mc²]] this includes matter as well.) | ||
==Second Law compared with other physical laws== | ==Second Law compared with other physical laws== | ||
| Line 205: | Line 203: | ||
The Second Law of Thermodynamics does not strictly apply to the following types of system: | The Second Law of Thermodynamics does not strictly apply to the following types of system: | ||
* [[Closed system]] - Exchanges energy, but not matter, with its surroundings | * [[Closed system]] - Exchanges energy, but not matter, with its surroundings | ||
| − | * | + | * Open system - Exchanges both matter and energy with its surroundings |
The boundary of any system can always be expanded to produce an [[isolated system]. | The boundary of any system can always be expanded to produce an [[isolated system]. | ||
Revision as of 19:51, October 2, 2016
The Second Law of Thermodynamics is a fundamental truth about the tendency towards disorder in the absence of intelligent intervention. This principle correctly predicts that heat will never flow from a cold body to a warmer one, unless forced to do so by a man-made machine. Or, as Isaac Asimov observed, "[t]he universe is constantly getting more disorderly."[1]
The Second Law of Thermodynamics is the result of the intrinsic uncertainty in nature, manifest in quantum mechanics, which is overcome only by intelligent intervention.
This law makes it impossible to build a perpetual motion machine - the increase in entropy inevitably derails the system even if energy remains constant.
The Second Law of Thermodynamics disproves the atheistic Theory of Evolution and Theory of Relativity, both of which deny a fundamental uncertainty to the physical world that leads to increasing disorder.
Contents
- 1 Entropy and disorder
- 2 Second Law compared with other physical laws
- 3 Probability and statistics
- 4 Application to molecular behavior
- 5 Reversibility and Irreversibility
- 6 Trend toward uniformity in the universe
- 7 The types of systems governed by the Law
- 8 Liberal abuse of the second law of thermodynamics
- 9 Creation Ministries International on the second law of thermodynamics and evolution
- 10 The 1st and 2nd law of thermodynamics and the universe having a beginning
- 11 The 1st and 2nd laws of thermodynamics, theism and the origins of the universe
- 12 See also
- 13 External links
- 14 References
Entropy and disorder
In this context "increasing disorder" means the decline in organization that occurs without intelligent intervention. Imagine your old room at your parent's house. Remember how easy it was to let the room turn into a uniform mess (disorder) and remember how hard it was to clean it up until it fit a specific, non-uniform design (order). Not cleaning up would always result in an increase of entropy in your room!
The flow of energy (by heat exchange) to places with lower concentrations is called the "heat flow."
The often-heard argument that this law disproves an eternal universe is true, because in that case maximum entropy would have been reached already. A counterargument to this would be to suggest that the universe is still in the process of approaching maximum entropy.
There are many different ways if stating the second law of thermodynamics. An alternative statement of the law is that heat will tend not to flow from a cold body to a warmer one without intelligent intervention, or work, being done, as in the case of a refrigerator. Other statements include that it is impossible for an engine to convert heat perfectly (I.e. at 100% efficiency) into work. These statements are qualitative and stating the second law in terms of entropy makes the law quantitative.
This law makes it impossible to build a perpetual motion machine - the increase in entropy inevitably derails the system even if its energy remains constant (as described by the first law of thermodynamics).
The second law can be expressed mathematically as:

where  is the rate of change of entropy of the universe with respect to time.
is the rate of change of entropy of the universe with respect to time.
Another way of viewing the second law is in terms of probability. In nature there is no one to clean up the universe, only chances. The chance of something becoming orderly is essentially zero, which it is a certainty that things will become more disorderly.
On a universal scale a tidy room would be a universe which has pockets of above average concentrations of energy (if you - incorrectly - assume relativity E=mc² this includes matter as well.)
Second Law compared with other physical laws
Thermodynamics occupies an unusual place in the world of science, particularly at the high school and undergraduate levels. The second law is the one that is especially peculiar. (In fact, the other laws are comparatively mundane. The first law is just a statement that heat is a form of energy, and that energy, whether in the form of heat or not, is conserved. This was a very nontrivial result at first, but, with the understanding of heat and temperature that later developed, it's quite unremarkable. The third law is a statement that absolute zero can't be reached by any finite number of Carnot cycles. While true, its significance pales in comparison to that of the second law.)
Perhaps what makes the second law so remarkable is that it describes irreversible phenomena. In particular, it describes the observed fact that heat energy, in bodies that are not being externally manipulated by compression, etc., flows only from a warmer body to a cooler one. When a warmer body is placed in contact with a cooler one, heat energy will flow (always perserving total energy, of course) from the warmer one to the cooler one. The warmer one will cool off as it releases its energy, and the cooler one will warm up. This process will continue until the two bodies reach the same temperature, or "thermal equilibrium".
Until the development of statistical mechanics, no one knew why this was so, or what temperature actually meant. What was known was simply that a body with a higher temperature would send heat to a body with a lower temperature, no matter what the bodies were made of.
The fact that this kind of heat flow is irreversible makes the whole field of thermodyamics lie outside of the realm of classical Newtonian mechanics or Relativistic mechanics. In Newtonian or Relativistic mechanics, every phenomenon can go in reverse order. The catchy phrase "arrow of time" (or "time's arrow") was coined by Arthur Eddington to denote this one-way behavior not shared by other theories of physics.[2]
Prior to the insight of quantum mechanics, which established the fundamental underlying uncertainty in the universe, the field of statistical mechanics attributed the increase in entropy to the statistical tendencies of huge aggregates of particles at the molecular or atomic level. While Newtonian and Relativistic mechanics can, in principle, precisely describe assemblages of any number of particles, in practice they are not directly applied to the behavior of bulk material. That is, they are not applied to a number of particles on the order of Avogadro's number.
Statistical mechanics was developed in the 19th century prior to quantum mechanics, so rather than attributing the Second Law to the fundamental uncertainty in nature, statistical mechanics bases its models on assumptions concerning the statistical behavior of large numbers of particles.
Probability and statistics
If you flipped a coin 20 times and it came up heads each time, you would consider that to be a remarkable occurrence. (Perhaps so much so that you would inspect the coin to be sure it didn't have heads on both sides.) If you tried it and got tttthtththhhththhthh, you would probably not consider it remarkable. Yet each of these outcomes is equally probable: about 1 in 106. If you shuffled a deck of cards and found them all in exact order from the 2 of clubs to the ace of spades, you would consider that to be very remarkable. But if you got the distribution shown in the illustration on page 314 of Alfred Sheinwold's 5 Weeks to Winning Bridge, you would probably consider it just "random". Yet each of these orderings has the same probability of occurring—1 in 52 factorial, which is about 1066.[3]
For small sets such as coin tosses or card shufflings, what constitutes "random" vs. "well ordered" is in the eye of the beholder. If a room in your house started in a state that most people would consider "neat and tidy", and you went into that room every day, picked up a random obect, and threw it against a random wall, after month most people would consider the room a mess. But, once again, this is hard to quantify. The kinds of statistical analyses that are required for the study of thermodynamics have to be much more careful than this. The kind of folksy quotations in popular articles about messy rooms, as in the Isaac Asimov quote, may sell magazines, but they don't shed much light on how thermodynamics works.
What is needed is an analysis of aggregate properties, not individual items. We need to quantify the results. For the case of the coin toss, we might ask how many times we got heads. The probabilities can be worked out; they are a "Gaussian distribution", also known as a "bell curve". The probability of heads 0 times out of 20 is 1 in 1048576. Getting heads exactly 1 time is .00002, and so on, as shown in this table. Notice that getting heads exactly 10 times is the most probable outcome, but its probability is still only 18%. If we did the experiment a larger number of times, the probability of exactly 50% heads would still be higher than any other, but it would be quite small. What is important is the probability of getting a certain number of heads or less.
| Number of times it
comes up heads |
Probability of that
exact number |
Probability of that
number or less |
|---|---|---|
| 0 | 10-6 | 10-6 |
| 1 | .00002 | .00002 |
| 2 | .00002 | .0002 |
| 3 | .0011 | .0013 |
| 4 | .0046 | .0059 |
| 5 | .0148 | .0207 |
| 6 | .0370 | .0577 |
| 7 | .0739 | .1316 |
| 8 | .1201 | .2517 |
| 9 | .1602 | .4119 |
| 10 | .1762 | .5881 |
| 11 | .1602 | .7483 |
| 12 | .1201 | .8684 |
| 13 | .0739 | .9423 |
| 14 | .0370 | .9793 |
| 15 | .0148 | .9941 |
| 16 | .0046 | .9987 |
| 17 | .0011 | .9998 |
| 18 | .00002 | .99998 |
| 19 | .00002 | .99999 |
| 20 | 10-6 | 1.000 |
With really large numbers, the probability of any particular outcome is vanishingly small; the only sensible measure is the accumulated probability, or the probability density, measured in a way that doesn't involve individual outcomes.
When dealing with thermodynamics, we are dealing with the statistical aggregate behavior of macroscopic pieces of matter, so we have to increase the number of items from 10, or 52, to something like Avogadro's number. So the number of possible situations, instead of being 106 or 1066, is something like 10Avogadro's number, that is, 101023. The enormity of such a number makes a huge amount of difference.
- You can't ask any questions about individual items—air molecules don't have labels like "Jack of Diamonds". You can only ask questions about the aggregate behavior of macroscopic pieces of space.
- While the probabilities of certain outcomes can be mathematically calculated, they are so small that, as a practical matter, we can say that they do not occur. People sometimes like to say things like "The second law of thermodynamics means that it is very unlikely that heat will travel from a colder object to a warmer one." That's a fallacious way of thinking about it. It is a statistical impossibility—it just doesn't occur.
- An example is the question of how likely it is that all the air molecules in a room will move to one corner, asphyxiating everyone.[4] This is sometimes worked out in physics classes. But the conclusion has to be that this occurrence, or anything remotely resembling it, might have a probability on the order of 1 in 101023—it just doesn't happen.
Application to molecular behavior
The development of the kinetic theory of gases, statistical mechanics, and thermodynamics revolutionized 19th century physics. It was recognized that, while we can't analyze the behavior of every molecule, we can analyze the statistical behavior of macroscopic assemblages. When gas molecules collide, they can transfer energy in a manner that leads to the principle of equipartition of energy. This, plus the constraints on conservation of the total energy, leads to the Maxwell-Boltzmann distribution of molecular energies. From this, one can deduce the properties of volume, pressure, and temperature, leading to Boyle's law and Charles' law, among others. Temperature was found to be just the average energy per molecule. (Actually, the average energy per "degree of freedom".) The equipartition principle was found to give an explanation of the fact that, when two bodies are in contact, they exchange energy in a way that makes the warmer body get cooler and the cooler body get warmer, always in accordance with conservation of energy.
Example of the Statistical nature of the Second Law
To see this divide a container two and suppose that 1 half contains 20 molecules and the other none. What we expect to happen is for the molecules to spread out and have roughly half (10) in each half of the container. We can calculate the number of micro-states,  , which correspond to each macro-state:
, which correspond to each macro-state:
| Macro-state | Number of Micro-states, 
|
Entropy 
|
|---|---|---|
| All particles in one half | 1 | 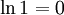
|
| 1 in left and 19 in right | 20 | 
|
| 10 in left and 10 in right | 184756 | 
|
Note that the Boltzmann constant has taken a value of 1 to simplify the maths.
So the state that we would expect to find the system in, the last one, has the highest entropy. However, the system could be in this state (10 in left, 10 in the right) and, just by chance, all the molecules could make their way to the left hand side of the box. This corresponds to a decrease of entropy. This example could be expanded up to a room, so why do we never see all the air in a room suddenly move to one end? The reason is that it is so unlikely, perhaps less than  , than it practically never occurs[5]. Hence it may be assumed that for most systems entropy never decreases. This is known as the fluctuation theorem.
, than it practically never occurs[5]. Hence it may be assumed that for most systems entropy never decreases. This is known as the fluctuation theorem.
Reversibility and Irreversibility
Reversibility is a theoretical concept related to the Second Law of Thermodynamics. A process is reversible if the net heat and work exchange between the system and the surroundings is zero for the process running forwards and in reverse. This means the process does not generate entropy. In reality, no process is completely reversible. Irreversibility is a quantity sometimes called "lost work" and is equal to the difference between a process' actual work and reversible work. Irreversibility is also equal to a process' entropy generation multiplied by a reference temperature.
Trend toward uniformity in the universe
The universe will always become increasingly uniform, that is: heat will spread until the entire universe has the temperature and energy level (in an isolated system heat will always spread from a place where there is a lot of heat to a place where there is less until balance is achieved), forces will continue to work until a universal balance has been achieved.
In this final state the universe is one uniform space where nothing happens and no work (moving something) can be done since there are no above average concentrations of energy left. This state is called maximum entropy and is said to be in perfect disorder (although intuitively its uniformity would seem to be a state of perfect order) because it has become impossible to determine what happened in the past. i.e. There are an infinite number of ways (histories of the universe) maximum entropy could have been reached.
The types of systems governed by the Law
There is only one type of system that the Second Law of Thermodynamics applies to: an isolated system. An isolated system is one that does not exchange matter or energy with its surroundings.
The Second Law of Thermodynamics does not strictly apply to the following types of system:
- Closed system - Exchanges energy, but not matter, with its surroundings
- Open system - Exchanges both matter and energy with its surroundings
The boundary of any system can always be expanded to produce an [[isolated system].
Liberal abuse of the second law of thermodynamics
It has become common in recent years for environmentalists to claim that the second law of thermodynamics implies limits to economic growth. Their reasoning is that because free energy in resources such as oil decreases with time, then economic growth can only be finite. However, this simplistic liberal reasoning ignores the non-zero sum nature of free market economics, whereby improvements in technology deliver gains for all at no further cost. Indeed, one of the most vital economic goods, knowledge, or more generally information, can be said to be free from thermodynamic limitations entirely.[6] Liberals also vastly exaggerate the limitations that natural resources impose on human economies. Some estimate that the Earth can harbor 100 billion people. God Himself gives His explicit assurance that the Earth will be generous as long as the human race exists in Genesis: "And God blessed them, and God said unto them, Be fruitful, and multiply, and replenish the earth, and subdue it: and have dominion over the fish of the sea, and over the fowl of the air, and over every living thing that moveth upon the earth." (Gen. 1:28, KJV)
Creation Ministries International on the second law of thermodynamics and evolution
See also: Creation Ministries International on the second law of thermodynamics and evolution
Creation Ministries International has a great wealth of information on why the second law of thermodynamics is incompatible with the evolutionary paradigm.
Some of their key resources on this matter are:
The main argument againstevolution using the second law of thermodynamics is that evolution requires a decrease in entropy (disorder). However, the second law of thermodynamics states that entropy increases, so the two are contradictory. Evolutionists and other scientists claim that these resources misrepresent the Second Law of Thermodynamics, negating the fact the earth is not an isolated system (energy is added from the sun for example). They also claim that they disregard the statistical nature of the law, in that entropy can decrease, but is so unlikely that no one ever sees this and it can be assumed to never happen.
The 1st and 2nd law of thermodynamics and the universe having a beginning
See also: Atheism and the origin of the universe
According to Ohio State University professor Patrick Woodward, the First Law of Thermodynamics "simply states that energy can be neither created nor destroyed (conservation of energy)."[7]
The Christian apologetics website Why believe in God? declares about the Second Law of Thermodynamics:
| “ | The second law of thermodynamics, or the law of increased entropy, says that over time, everything breaks down and tends towards disorder - entropy! Entropy is the amount of UNusable energy in any systems; that system could be the earth's environment or the universe itself. The more entropy there is, the more disorganisation and chaos.
Therefore, if no outside force is adding energy to an isolated system to help renew it, it will eventually burn out (heat death). This can be applied to a sun as well as a cup of tea - left to themselves, both will grow cold. You can heat up a cold tea, you cannot heat up a cold sun. NOTE: when a hot tea in an air tight room goes cold (loses all it's energy) not only do we NOT expect the process to reverse by natural causes (ie. the tea will get hot again), but both room temp and tea temp will be equal. Keep that in mind as you read the next paragraph. Look at it like this, because the energy in the universe is finite and no new energy is being added to it (1st law), and because the energy is being used up (2nd law), the universe cannot be infinite. If our universe was infinite but was using up a finite supply of energy, it would have suffered 'heat death' a long time ago! If the universe was infinite all radioactive atoms would have decayed and the universe would be the same temperature with no hot spots, no bright burning stars. Since this is not true, the universe must have begun a finite time ago.[8] |
” |
Thus, the First Law of Thermodynamics and the Second Law of Thermodynamics suggests that the universe had a beginning.[9] This argument is similar to the argument for the big bang, that the universe is expanding now, so in the past it must have been smaller, and since there is a limit to how small the universe can be, it points to a beginning. In this argument, it is entropy that has a lower limit.
However, the statistical nature of the second law mean that it is not firmly true. If the universe is infinite in time, then eventually all possibilities, no matter how small, are played out. In this way, the universe could reach maximum entropy and then happen, by chance, to return to a low entropy state. This would happen an infinite number of times. Hence we could be in the process of entropy increasing and the universe need nor have a beginning.
The 1st and 2nd laws of thermodynamics, theism and the origins of the universe
In the articles below, theists point out that the First Law of Thermodynamics and the Second Law of Thermodynamics point to the universe having a divine origin:
- Evidence for the Supernatural Creation of the Universe by Patrick R. Briney, Ph.D.
- The Laws of Thermodynamics Don't Apply to the Universe! by Jeff Miller, Ph.D.
- God and the Laws of Thermodynamics: A Mechanical Engineer’s Perspective by Jeff Miller, Ph.D.
- If God created the universe, then who created God? by Jonathan Sarfati, Ph.D.
See also
- Thermodynamics
- Entropy
- Essay:Commentary on Conservapedia's article on the second law of thermodynamics
- Genetic entropy
External links
References
- ↑ Isaac Asimov continued to explain that "[v]iewed that way, we can see the second law all about us. We have to work to straighten a room, and when left to itself it becomes a mess again very quickly and very easily. Even if we never enter it, it becomes dusty and musty. How difficult to maintain houses, and machinery, and our bodies in perfect working order: how easy to let them deteriorate. In fact, all we have to do is nothing, and everything deteriorates, collapses, breaks down, wears out, all by itself -- and that is what the second law is all about.” Isaac Asimov, Smithsonian Institute Journal, June 1970, p. 6. Put in physics terminology, the Second Law states that the entropy of an isolated or closed system never decreases.
- ↑ The Nature of the Physical World, Arthur Eddington, MacMillan, 1929, ISBN 0-8414-3885-4
- ↑ Actually, we're ignoring the fact that bridge players sort their cards by suit, and the diagram shows the result of the sorting.
- ↑ Actually, conservation of momentum requires that we consider half the molecules going to one corner and the other half to the opposite corner.
- ↑ Hugh D. Young and Roger A. Freedman. University Physics with Modern Physics (in English). San Francisco: Pearson.
- ↑ [1] Discovery Institute cofounder and futurist George F. Gilder put it eloquently as follows: Gone is the view of a thermodynamic world economy, dominated by "natural resources" being turned to entropy and waste by human extraction and use. Once seen as a physical system tending toward exhaustion and decline, the world economy has clearly emerged as an intellectual system driven by knowledge.
- ↑ 1st Law of Thermodynamics, Ohio State University, Professor Pat Woodward (teaches for the Department of Chemistry & Biochemistry [2])
- ↑ Is the Universe Infinite? Past beliefs and implications, Why believe in God? website
- ↑
- CAN LAWS OF SCIENCE EXPLAIN THE ORIGIN OF THE UNIVERSE?
- Evidence for the Supernatural Creation of the Universe by Patrick R. Briney, Ph.D.
- The Laws of Thermodynamics Don't Apply to the Universe! by Jeff Miller, Ph.D.
- God and the Laws of Thermodynamics: A Mechanical Engineer’s Perspective by Jeff Miller, Ph.D.
- If God created the universe, then who created God? by Jonathan Sarfati, Ph.D.
- Has the universe always existed?
