Difference between revisions of "Bohr atom"
DavidB4-bot (Talk | contribs) (→Structure: Spelling/Grammar Check, typos fixed: a electron → an electron) |
m (→References: cat) |
||
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Bohr's model of the atom''' was the first model that combined [[Quantum | + | '''Bohr's model of the atom''' was the first model that combined [[Quantum mechanics|quantum]] and classical theories of physics in an attempt to explain the observations surrounding the emission spectra of atoms. |
==Background== | ==Background== | ||
| Line 9: | Line 9: | ||
==Structure== | ==Structure== | ||
| − | The Bohr model contains a | + | The Bohr model contains a centre nucleus containing [[protons]] and [[neutrons]]. Electrons orbit the nucleus in specific orbits with a fixed radius, with each orbit having a [[Quantum number|principle atomic number]] of n = 1, 2, 3 with successive numbers indicating an orbital with a higher radius and electron energy, and with the energy gap between the first and second being larger than the second and third and so on. Electrons would initially fill the lower energy orbits before the higher ones, this formation being known as the ground state. |
If an electron in the ground state absorbed energy it would be able to be promoted to a higher energy orbit. After time it would fall back to its original state releasing a photon of light with energy corresponding to the gap between the two levels. The electron did not necessarily fall straight back, for example it could jump from n=1 to n=3 and either fall back from n=3 to n=1 or from n=3 to n=2 and then n=2 to n=1. As the energy levels between each level were different this allowed for a number of wavelengths to be emitted if a sample was exposed to a large energy source, however as the values were fixed only specific wavelengths were allowed hence explaining the discrete lines on the emission spectra of elements. | If an electron in the ground state absorbed energy it would be able to be promoted to a higher energy orbit. After time it would fall back to its original state releasing a photon of light with energy corresponding to the gap between the two levels. The electron did not necessarily fall straight back, for example it could jump from n=1 to n=3 and either fall back from n=3 to n=1 or from n=3 to n=2 and then n=2 to n=1. As the energy levels between each level were different this allowed for a number of wavelengths to be emitted if a sample was exposed to a large energy source, however as the values were fixed only specific wavelengths were allowed hence explaining the discrete lines on the emission spectra of elements. | ||
| − | Bohr | + | Bohr modelled his atom on the emission spectrum of hydrogen. The [[element]]al atom of hydrogen at ground state contains an electron in the first electron orbit, n=1. When the atom is excited the electron can jump into a higher state including energy levels n=2, n=3, n=4, n=5, n=6, and then would fall back to its ground state. Bohr calculated a number of transitions were possible, including: |
* Paschen Series - wavelength in the [[Infrared|IR]] range, when an electron falls to n=3 from a higher orbit | * Paschen Series - wavelength in the [[Infrared|IR]] range, when an electron falls to n=3 from a higher orbit | ||
* [[Balmer series|Balmer Series]] - wavelength in the visible range, when an electron falls to n=2 from a higher orbit, specifically | * [[Balmer series|Balmer Series]] - wavelength in the visible range, when an electron falls to n=2 from a higher orbit, specifically | ||
| Line 23: | Line 23: | ||
** n=8 to n=2 - 388.9 nm (violet) | ** n=8 to n=2 - 388.9 nm (violet) | ||
* Lymann Series - wavelength in the [[Ultraviolet|UV]] range, when an electron falls from n=2 to n=1 | * Lymann Series - wavelength in the [[Ultraviolet|UV]] range, when an electron falls from n=2 to n=1 | ||
| + | |||
| + | ==Mathematics== | ||
| + | |||
| + | The model is based on [[classical physics]] and ignores radiation emitted by the [[electron]] due to its acceleration. It also supposes that [[electrons]] are only allowed to have certain values ofr their [[angular momentum]], or that angular momentum is '''quantised'''. It correctly predicts, by chance, the relationship beween different levels and their radius and energy. These radius relationship is: | ||
| + | |||
| + | <math> | ||
| + | r_n = \epsilon_0 \frac{n^2 h^2}{\pi me^2} | ||
| + | </math> | ||
| + | |||
| + | where | ||
| + | |||
| + | :<math>\epsilon_0</math> is the permittivity of free space, equal to <math>8.854187817 \times 10^{-12} \, \mbox{F} \, \mbox{m}^{-1}</math><ref>http://physics.nist.gov/cgi-bin/cuu/Value?ep0</ref> | ||
| + | :<math>n</math> is the n<sup>th</sup> energy level | ||
| + | :<math>h</math> is [[Planck's constant]] | ||
| + | :<math>m</math> is the mass of an [[electron]] | ||
| + | :<math>e</math> is the elementary charge (charge on an electron) | ||
| + | |||
| + | |||
| + | The relationship for the energy levels is: | ||
| + | |||
| + | <math> | ||
| + | E_n = - \frac{hcR}{n^2} | ||
| + | </math> | ||
| + | |||
| + | where | ||
| + | |||
| + | :<math>c</math> is the [[speed of light]] | ||
| + | :<math>R</math> is the Ryberg constant, which is: | ||
| + | |||
| + | <math> | ||
| + | R= \frac{me^4}{8 \epsilon_0^2 h^3 c} | ||
| + | </math> | ||
| + | |||
| + | with all constants being the same as above.<ref>{{cite book | ||
| + | |author=Hugh D. Young and Roger A. Freedman | ||
| + | |title=University Physics with Modern Physics | ||
| + | |publisher=Pearson | ||
| + | |location=San Francisco | ||
| + | |isbn= | ||
| + | |pages= | ||
| + | |quote= | ||
| + | |language=English}}</ref> | ||
| + | |||
| + | ===Derivation=== | ||
| + | |||
| + | The most important part is the quantisation of [[angular momentum]], <math>L_n</math> as: | ||
| + | |||
| + | <math>L_n = mv_n r_n = n \hbar</math> | ||
| + | |||
| + | where | ||
| + | |||
| + | :<math>v_n</math> is the [[speed]] of the [[electron]] in the n<sup>th</sup> level | ||
| + | :<math>\hbar</math> is the reduced Planck constant | ||
| + | |||
| + | This can be done since [[Planck's constant]] has the same units as [[angular momentum]] | ||
| + | |||
| + | By considering [[Coulomb's Law|Coulomb's law]], the electrostatic [[force]] between the electron and the [[proton]] and the [[acceleration]] of the electron undergoing circular motion we find: | ||
| + | |||
| + | <math> | ||
| + | \frac{1}{4 \pi \epsilon_0} \frac{e^2}{r_n^2}= \frac{mv_n^2}{r^n} | ||
| + | </math> | ||
| + | |||
| + | Solving this equation with the above determines <math>r_n</math> as above and: | ||
| + | |||
| + | <math> | ||
| + | v_n = \frac{1}{\epsilon_0} \frac{e^2}{2nh} | ||
| + | </math> | ||
| + | |||
| + | for the speed. | ||
| + | The [[energy]] of each level can be found as the sum of the [[kinetic energy|kinetic]] and potential energies: | ||
| + | |||
| + | <math> | ||
| + | E_n = \frac{1}{2} m v_n^2 - \frac{e^2}{2 \pi \epsilon_0 r_n^2} | ||
| + | </math> | ||
| + | |||
| + | Substituting in the above gives the desired result: | ||
| + | |||
| + | <math> | ||
| + | E_n = \frac{me^4}{8 \epsilon_0^2 h^2} \frac{1}{n^2} | ||
| + | </math> | ||
==Limitations== | ==Limitations== | ||
| Line 28: | Line 108: | ||
Although providing a revolutionary approach to scientific understanding of the atom the Bohr model was limited in several aspects: | Although providing a revolutionary approach to scientific understanding of the atom the Bohr model was limited in several aspects: | ||
* Although it allowed calculations of the energy orbits around hydrogen these couldn't be applied to other atoms, it was later found this was because different atoms have different electron energy levels | * Although it allowed calculations of the energy orbits around hydrogen these couldn't be applied to other atoms, it was later found this was because different atoms have different electron energy levels | ||
| − | * It could not explain the concept of doublets, these were shown to be due to | + | * It could not explain the concept of doublets, these were shown to be due to sub-shells within energy levels |
* It contained electrons orbiting with fixed radii, this was later shown to be false by the [[Heisenberg uncertainty principle]], an effect of which is that the probability of an electron appearing in a location can only be predicted, its path cannot be mapped | * It contained electrons orbiting with fixed radii, this was later shown to be false by the [[Heisenberg uncertainty principle]], an effect of which is that the probability of an electron appearing in a location can only be predicted, its path cannot be mapped | ||
| + | ==References== | ||
| + | {{Reflist}} | ||
| + | |||
| + | [[Category:History of Science]] | ||
| + | [[Category:Atomic Chemistry]] | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
Latest revision as of 12:22, April 9, 2017
Bohr's model of the atom was the first model that combined quantum and classical theories of physics in an attempt to explain the observations surrounding the emission spectra of atoms.
Background
The theory that preceded Bohr's model was the Rutherford model of the atom, which postulated that an atom contained a core nucleus and a number of electrons orbiting around it. This model was somewhat limited, and couldn't explain the emission spectra observed from a hydrogen lamp.
Max Planck had previously put forward his theory of quantum physics, in which energy could only be absorbed and emitted in discrete packets or quanta. Bohr applied this theory to his model of the atom, postulating that electrons could only exist in certain fixed energy orbits around the nucleus, and that they could absorb and emit specific amounts of energy to progress between.
Structure
The Bohr model contains a centre nucleus containing protons and neutrons. Electrons orbit the nucleus in specific orbits with a fixed radius, with each orbit having a principle atomic number of n = 1, 2, 3 with successive numbers indicating an orbital with a higher radius and electron energy, and with the energy gap between the first and second being larger than the second and third and so on. Electrons would initially fill the lower energy orbits before the higher ones, this formation being known as the ground state.
If an electron in the ground state absorbed energy it would be able to be promoted to a higher energy orbit. After time it would fall back to its original state releasing a photon of light with energy corresponding to the gap between the two levels. The electron did not necessarily fall straight back, for example it could jump from n=1 to n=3 and either fall back from n=3 to n=1 or from n=3 to n=2 and then n=2 to n=1. As the energy levels between each level were different this allowed for a number of wavelengths to be emitted if a sample was exposed to a large energy source, however as the values were fixed only specific wavelengths were allowed hence explaining the discrete lines on the emission spectra of elements.
Bohr modelled his atom on the emission spectrum of hydrogen. The elemental atom of hydrogen at ground state contains an electron in the first electron orbit, n=1. When the atom is excited the electron can jump into a higher state including energy levels n=2, n=3, n=4, n=5, n=6, and then would fall back to its ground state. Bohr calculated a number of transitions were possible, including:
- Paschen Series - wavelength in the IR range, when an electron falls to n=3 from a higher orbit
- Balmer Series - wavelength in the visible range, when an electron falls to n=2 from a higher orbit, specifically
- n=3 to n=2 - 656.3 nm (red)
- n=4 to n=2 - 486.1 nm (blue)
- n=5 to n=2 - 434.1 nm (violet)
- n=6 to n=2 - 410.2 nm (violet)
- n=7 to n=2 - 397.0 nm (violet)
- n=8 to n=2 - 388.9 nm (violet)
- Lymann Series - wavelength in the UV range, when an electron falls from n=2 to n=1
Mathematics
The model is based on classical physics and ignores radiation emitted by the electron due to its acceleration. It also supposes that electrons are only allowed to have certain values ofr their angular momentum, or that angular momentum is quantised. It correctly predicts, by chance, the relationship beween different levels and their radius and energy. These radius relationship is:

where
 is the permittivity of free space, equal to
is the permittivity of free space, equal to  [1]
[1]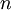 is the nth energy level
is the nth energy level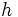 is Planck's constant
is Planck's constant is the mass of an electron
is the mass of an electron is the elementary charge (charge on an electron)
is the elementary charge (charge on an electron)
The relationship for the energy levels is:
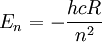
where
 is the speed of light
is the speed of light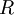 is the Ryberg constant, which is:
is the Ryberg constant, which is:

with all constants being the same as above.[2]
Derivation
The most important part is the quantisation of angular momentum, 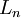 as:
as:

where
This can be done since Planck's constant has the same units as angular momentum
By considering Coulomb's law, the electrostatic force between the electron and the proton and the acceleration of the electron undergoing circular motion we find:

Solving this equation with the above determines  as above and:
as above and:
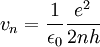
for the speed. The energy of each level can be found as the sum of the kinetic and potential energies:

Substituting in the above gives the desired result:
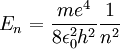
Limitations
Although providing a revolutionary approach to scientific understanding of the atom the Bohr model was limited in several aspects:
- Although it allowed calculations of the energy orbits around hydrogen these couldn't be applied to other atoms, it was later found this was because different atoms have different electron energy levels
- It could not explain the concept of doublets, these were shown to be due to sub-shells within energy levels
- It contained electrons orbiting with fixed radii, this was later shown to be false by the Heisenberg uncertainty principle, an effect of which is that the probability of an electron appearing in a location can only be predicted, its path cannot be mapped
References
- ↑ http://physics.nist.gov/cgi-bin/cuu/Value?ep0
- ↑ Hugh D. Young and Roger A. Freedman. University Physics with Modern Physics (in English). San Francisco: Pearson.
 is the
is the  is the reduced Planck constant
is the reduced Planck constant