Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle states that for any two quantum observables which do not commute, the product of the standard deviations of the measurements of those observables must be greater than or equal to a positive constant, related to Planck's constant ( ).
).
The most common example is of a particle's momentum  and its position
and its position  . Mathematically it is represented with this equation:
. Mathematically it is represented with this equation:
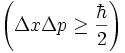 .
.
This means that we can never measure both the position and momentum of a particle simultaneously with arbitrary precision. The more precisely we wish to measure one observable, the less precisely we can measure the other at that time.
Contrary to popular belief, this is not merely a measurement issue. While it is often stated that it is not possible to know the precise position and momentum of a particle at the same time, this is misleading; it implies that the particle has precisely defined position and momentum, but that information is unavailable to us. In fact, the Uncertainty Principle tells us that a particle cannot have precisely defined position and momentum simultaneously.
A similar example is time and energy, the product of which also has a lower limit. Quantum fluctuations are a result of this, where for short time, there is enough energy in "empty space" to create a pair of particle and antiparticle, such as electron and positron. Although this appears to be a strange or extreme process, it is the only one to explain some properties of black holes. Laser physics with ultrashort laser pulses is another field, where the limit in the product of time and energy plays an important role.