Photon
A photon is a particle of light. The term was coined by the American physical chemist Gilbert Newton Lewis.[1]
Photons are bosons, that is, particles that have integer spin. In quantum field theory, photons are the mediators of the electromagnetic force.
Historical development
Since Newton's publication of his Opticks in 1704, his corpuscle theory of light dominated the world of physics. However, this changed in the 19th century with experiments done by Augustin-Jean Fresnel and Thomas Young, which supported Christian Huygens's wave theory. One of the proofs for this is that light experiences interference and diffraction, which would not be displayed if light were a classical particle. Furthermore, James Clerk Maxwell theoretical prediction of electromagnetic waves, together with Heinrich Hertz experiments led to conclude that light was an electromagnetic wave.
However, a phenomenon called the photoelectric effect led to a different conclusion. The photoelectric is the emission of electrons from a metal which is illuminated with light. This by itself is in no contradiction to the wave theory of light, but certain peculiarities of it were. For example, it was observed that no electrons were emitted unless the frequency of light was greater than a threshold frequency, regardless of how intense the light beam was. Also, the energy of the emitted electrons was dependent only on the light frequency, not in its intensity. Since the energy of a wave depends on its amplitude, which in turn is related to its intensity, this was in contradiction with the wave theory of light.
Albert Einstein solved this problem arguing that light is really a particle, which was called a photon, each of which carry an energy given by h*f, where h is Planck's constant and f is the frequency of the light. More intense light involves more photons, but the energy of each photon individually depends only on its frequency. This explained why there is a threshold frequency before a metal emits electrons: A single photon must be energetic enough to knock out an electron, and so increasing the intensity (sending more photons) will not solve the problem. Only increasing the frequency (increasing the energy per photon) will do.
In standard interpretations of quantum mechanics, light behaves both as a wave and as a particle. The existence of photons is now well accepted by the physics community, and its implication has gone well beyond the photoelectric effect.
Physics of photons
The Energy of a photon depends on its frequency and is given by the equation:

where f is the frequency of the electromagnetic wave and h is Planck's constant and has the numerical value of 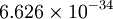 Js (Joules times second) or
Js (Joules times second) or 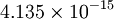 eV s (electron volts times second).
The momentum of a photon can be expressed as:
eV s (electron volts times second).
The momentum of a photon can be expressed as:

References
- ↑ Jonathan Sarfati. Should creationists accept quantum mechanics?. CMI. Retrieved on August 1, 2013. “Einstein called this Lichtquant or light quantum, but the American physical chemist Gilbert Newton Lewis (1875–1946) coined the term photon which stuck”