Difference between revisions of "Radioactivity"
(Put H. G. Wells' silliness in context.) |
DavidB4-bot (Talk | contribs) (→Beta Decay: Spelling/Grammar Check, typos fixed: is is → is) |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
As stated above, an alpha particle is the nucleus of a Helium atom, i.e., two protons and two neutrons. This arrangement means the alpha particle has a charge of +2, and an atomic mass of 4, the symbol for which is <math>{{}_2^4}He^{+2}</math>. | As stated above, an alpha particle is the nucleus of a Helium atom, i.e., two protons and two neutrons. This arrangement means the alpha particle has a charge of +2, and an atomic mass of 4, the symbol for which is <math>{{}_2^4}He^{+2}</math>. | ||
| − | For example, the most common [[isotope]] of Uranium is Uranium-238. | + | For example, the most common [[isotope]] of Uranium is Uranium-238. The [[mass number]],<ref>The mass number is the (integer) number of nucleons. It is very close to the [[atomic mass]] of the isotope measured in amu, because the masses of protons and neutrons are very close, and the deviations (due to [[E=mc²]]) are small. So the terms are often used nearly interchangeably.</ref> 238, is the sum of the number of protons and the number of neutrons. Since all Uranium atoms have 92 protons, there are 146 neutrons. The initial step in Uranium-238 decaying (eventually) into Lead-206 is an alpha decay: |
<center><math>{{}_{92}^{238}}U </math> → <math>{{}_2^4}He + {{}_{90}^{234}}Th</math></center> | <center><math>{{}_{92}^{238}}U </math> → <math>{{}_2^4}He + {{}_{90}^{234}}Th</math></center> | ||
| − | Note that the | + | Note that the proton count (92) is conserved, as is are the mass numbers (238). So the neutron count (146) is also balanced. No particles are created or destroyed. |
| − | + | This decay releases about 4.3 MeV of kinetic energy, in the form of the motion of the alpha particle. In chemical terms we would say that this decay is an exothermic reaction. The energy comes from the potential nuclear energy of the Uranium atom—the Uranium atom has a higher potential energy (by 4.3 Mev) than the sum of the potential energies of the Thorium and Helium atoms. A careful accounting of the atomic masses (remember, atomic mass is only approximately equal to mass number) will show that mass was lost, in accordance with [[E=mc²]]. | |
==Beta Decay== | ==Beta Decay== | ||
| Line 27: | Line 27: | ||
As with the alpha decay, notice that the particle count is again conserved. The energy released by this decay is 0.55 MeV. | As with the alpha decay, notice that the particle count is again conserved. The energy released by this decay is 0.55 MeV. | ||
| − | An interesting note about Strontium-90 is that | + | An interesting note about Strontium-90 is that is a synthetic isotope (meaning, it is not found naturally occurring, but must be manufactured) that is a by-product of nuclear weapons explosions. In the 1950s and 1960s, it was common to test nuclear weapons by exploding them in the very high upper atmosphere. Unfortunately, this resulted in a large amount of Strontium-90 particles that eventually settled back to earth, contaminating grass lands. The grasses were eaten by cattle, and the cattle were eaten by humans. |
Since Strontium is chemically very similar to Calcium (it is in the same column in the Periodic Table), any entrance of Strontium in the body will tend to replace the Calcium in our bones. In the case of Strontium-90, this meant that radioactive Strontium was now chemically bonded to our bones, and the 1970s saw a rise in bone cancer as a result. Fortunately, this type of testing was halted, and the [[half-life]] of Strontium-90 is a relatively short 28 years, meaning, at this point most of the synthetic, radioactive Strontium-90 produced by weapons testing has decayed out of the environment. | Since Strontium is chemically very similar to Calcium (it is in the same column in the Periodic Table), any entrance of Strontium in the body will tend to replace the Calcium in our bones. In the case of Strontium-90, this meant that radioactive Strontium was now chemically bonded to our bones, and the 1970s saw a rise in bone cancer as a result. Fortunately, this type of testing was halted, and the [[half-life]] of Strontium-90 is a relatively short 28 years, meaning, at this point most of the synthetic, radioactive Strontium-90 produced by weapons testing has decayed out of the environment. | ||
==Energy of Radioactive Decay== | ==Energy of Radioactive Decay== | ||
| − | The energies associated with radioactive decay, at least on the single atom level, are very, very small. The energies are so small, in fact, we use a special unit called the Electron Volt (eV) rather than the | + | The energies associated with radioactive decay, at least on the single atom level, are very, very small. The energies are so small, in fact, we use a special unit called the Electron Volt (eV) rather than the traditional units of Joules or Btu or Foot-pounds. |
Just for a point of comparison, it takes about a minute to boil a cup of water in a 1000 Watt microwave. One Watt is equivalent to one Joule per second, so it takes 60,000 Joules of energy to boil a cup of water. But a single Joule of energy is the same as 6.24x10<sup>18</sup> eV! | Just for a point of comparison, it takes about a minute to boil a cup of water in a 1000 Watt microwave. One Watt is equivalent to one Joule per second, so it takes 60,000 Joules of energy to boil a cup of water. But a single Joule of energy is the same as 6.24x10<sup>18</sup> eV! | ||
| − | So even when a nuclear decay has an associated energy in the thousands (keV) or millions (MeV) of electron volts, we're still billions of factors away from having enough energy to boil a cup of water. The danger is not from a single atomic decay, but from many trillions of atomic decays occurring within rapid succession, in which case we do reach energies capable of producing serious burns on the skin. | + | So even when a nuclear decay has an associated energy in the thousands (keV) or millions (MeV) of electron volts, we're still billions of factors away from having enough energy to boil a cup of water. The danger is not from a single atomic decay, but from many trillions of atomic decays occurring within rapid succession, in which case we do reach energies capable of producing serious burns on the skin. |
==Antiquated notions== | ==Antiquated notions== | ||
| − | In the early decades of the | + | In the early decades of the 20th century, when it was a newly discovered phenomenon, there was much confusion about it, leading to some strange hypotheses. No less an intellectual than [[H. G. Wells]] wrote strange things about it. In his 1909 novel ''Tono-Bungay'', the narrator muses: |
:To my mind radio-activity is a real disease of matter. Moreover, it is a contagious disease. It spreads. You bring those debased and crumbling atoms near others and those too presently catch the trick of swinging themselves out of coherent existence. It is in matter exactly what the decay of our old culture is in society, a loss of traditions and distinctions and assured reactions. ...I am haunted by a grotesque fancy of the ultimate eating away and dry-rotting and dispersal of all our world. So that while man still struggles and dreams his very substance will change and crumble from beneath him. I mention this here as a queer persistent fancy. Suppose, indeed, that is to be the end of our planet; no splendid climax and finale, no towering accumulation of achievements, but just—atomic decay!<ref>Wells, H. G. (1909) [http://www.gutenberg.org/files/718/718-h/718-h.htm Tono-Bungay], online Project Gutenberg text; search for text string "real disease"</ref> | :To my mind radio-activity is a real disease of matter. Moreover, it is a contagious disease. It spreads. You bring those debased and crumbling atoms near others and those too presently catch the trick of swinging themselves out of coherent existence. It is in matter exactly what the decay of our old culture is in society, a loss of traditions and distinctions and assured reactions. ...I am haunted by a grotesque fancy of the ultimate eating away and dry-rotting and dispersal of all our world. So that while man still struggles and dreams his very substance will change and crumble from beneath him. I mention this here as a queer persistent fancy. Suppose, indeed, that is to be the end of our planet; no splendid climax and finale, no towering accumulation of achievements, but just—atomic decay!<ref>Wells, H. G. (1909) [http://www.gutenberg.org/files/718/718-h/718-h.htm Tono-Bungay], online Project Gutenberg text; search for text string "real disease"</ref> | ||
Revision as of 17:25, August 11, 2016
Radioactivity is the emission of high energy particles through the natural phenomenon of the decay of unstable isotopes of chemical elements into more stable forms, which are called daughter products. This type of emission is generally called nuclear radiation.
The most common types of nuclear radiation are alpha and beta radiation, and the processes for each are respectively alpha decay and beta decay.[1] There can also be gamma radiation associated with a nuclear decay. Alpha particles are helium nuclei (two protons and two neutrons); beta particles are high-energy electrons; gamma rays are high-energy photons. Alpha particles can normally be stopped by a sheet of paper or healthy human skin. Beta particles and gamma rays can penetrate one's body to cause great harm. Gamma radiation is also a form of electro-magnetic radiation, like X-rays or visible light. (Contemporary jargon refers to alpha and beta particles and gamma rays, though quantum mechanics makes the two actually the same.)
All forms of radioactivity follow the fundamental rules of mass and energy balance.
Contents
Alpha Decay
- Main article: Alpha decay
As stated above, an alpha particle is the nucleus of a Helium atom, i.e., two protons and two neutrons. This arrangement means the alpha particle has a charge of +2, and an atomic mass of 4, the symbol for which is  .
.
For example, the most common isotope of Uranium is Uranium-238. The mass number,[2] 238, is the sum of the number of protons and the number of neutrons. Since all Uranium atoms have 92 protons, there are 146 neutrons. The initial step in Uranium-238 decaying (eventually) into Lead-206 is an alpha decay:
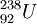 →
→ 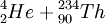
Note that the proton count (92) is conserved, as is are the mass numbers (238). So the neutron count (146) is also balanced. No particles are created or destroyed.
This decay releases about 4.3 MeV of kinetic energy, in the form of the motion of the alpha particle. In chemical terms we would say that this decay is an exothermic reaction. The energy comes from the potential nuclear energy of the Uranium atom—the Uranium atom has a higher potential energy (by 4.3 Mev) than the sum of the potential energies of the Thorium and Helium atoms. A careful accounting of the atomic masses (remember, atomic mass is only approximately equal to mass number) will show that mass was lost, in accordance with E=mc².
Beta Decay
- Main article: Beta decay
As stated above, a beta particle is an electron. This arrangement means the beta particle has a charge of -1, and an atomic mass of 0, the symbol for which is  .
.
Strontium-90 undergoes beta decay to form Yttrium-90 in the following decay reaction:
 →
→ 
As with the alpha decay, notice that the particle count is again conserved. The energy released by this decay is 0.55 MeV.
An interesting note about Strontium-90 is that is a synthetic isotope (meaning, it is not found naturally occurring, but must be manufactured) that is a by-product of nuclear weapons explosions. In the 1950s and 1960s, it was common to test nuclear weapons by exploding them in the very high upper atmosphere. Unfortunately, this resulted in a large amount of Strontium-90 particles that eventually settled back to earth, contaminating grass lands. The grasses were eaten by cattle, and the cattle were eaten by humans.
Since Strontium is chemically very similar to Calcium (it is in the same column in the Periodic Table), any entrance of Strontium in the body will tend to replace the Calcium in our bones. In the case of Strontium-90, this meant that radioactive Strontium was now chemically bonded to our bones, and the 1970s saw a rise in bone cancer as a result. Fortunately, this type of testing was halted, and the half-life of Strontium-90 is a relatively short 28 years, meaning, at this point most of the synthetic, radioactive Strontium-90 produced by weapons testing has decayed out of the environment.
Energy of Radioactive Decay
The energies associated with radioactive decay, at least on the single atom level, are very, very small. The energies are so small, in fact, we use a special unit called the Electron Volt (eV) rather than the traditional units of Joules or Btu or Foot-pounds.
Just for a point of comparison, it takes about a minute to boil a cup of water in a 1000 Watt microwave. One Watt is equivalent to one Joule per second, so it takes 60,000 Joules of energy to boil a cup of water. But a single Joule of energy is the same as 6.24x1018 eV!
So even when a nuclear decay has an associated energy in the thousands (keV) or millions (MeV) of electron volts, we're still billions of factors away from having enough energy to boil a cup of water. The danger is not from a single atomic decay, but from many trillions of atomic decays occurring within rapid succession, in which case we do reach energies capable of producing serious burns on the skin.
Antiquated notions
In the early decades of the 20th century, when it was a newly discovered phenomenon, there was much confusion about it, leading to some strange hypotheses. No less an intellectual than H. G. Wells wrote strange things about it. In his 1909 novel Tono-Bungay, the narrator muses:
- To my mind radio-activity is a real disease of matter. Moreover, it is a contagious disease. It spreads. You bring those debased and crumbling atoms near others and those too presently catch the trick of swinging themselves out of coherent existence. It is in matter exactly what the decay of our old culture is in society, a loss of traditions and distinctions and assured reactions. ...I am haunted by a grotesque fancy of the ultimate eating away and dry-rotting and dispersal of all our world. So that while man still struggles and dreams his very substance will change and crumble from beneath him. I mention this here as a queer persistent fancy. Suppose, indeed, that is to be the end of our planet; no splendid climax and finale, no towering accumulation of achievements, but just—atomic decay![3]
This is not to say that radioactivity isn't dangerous, or that radioactive contamination isn't a tricky problem.
Notes and references
- ↑ http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/radact.html
- ↑ The mass number is the (integer) number of nucleons. It is very close to the atomic mass of the isotope measured in amu, because the masses of protons and neutrons are very close, and the deviations (due to E=mc²) are small. So the terms are often used nearly interchangeably.
- ↑ Wells, H. G. (1909) Tono-Bungay, online Project Gutenberg text; search for text string "real disease"