Radiometric dating
Radiometric dating is using a radioactive process to determine the age of an item.[1] Radiometric dating is mostly used to determine the age of rocks (though in the case of Radiocarbon dating can date wood, cloth, skeletons, and other organic material), and relies on the well-established function for exponential decay 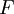


 . Different radiometric techniques are calibrated in different ways; for example, radiocarbon dating is calibrated by dating wood that has been dated through the process of dendrochronology.
. Different radiometric techniques are calibrated in different ways; for example, radiocarbon dating is calibrated by dating wood that has been dated through the process of dendrochronology.
Contents
Key assumptions
Ratios of materials
Radiometric dating works on the assumption that there is some natural level of radioactive material and material that it will decay to in nature. For example, with Radiocarbon dating, this is carbon 14 and Nitrogen 14. The level of carbon 14 in the environment is stable with the two exceptions of bomb carbon and burning of ancient fuels (increasing the amount of carbon 12 in the environment). Both of these can be removed by using wood from 1890 - prior to the widespread use of fossil fuels and well before the creation of the nuclear bomb.
With substances such as uranium, there is no creation of new uranium in the history of the world (with the exception of the very recent phenomenon of nuclear enrichment - which never gets back into ancient rocks so this is moot).
Younge Earth Creationists assert that the rate of formation of carbon 14 changed with creation and the flood leading to incorrect results for dating.
Rate of decay
The rate of decay of a radioactive material is constant. This is based upon knowledge of quantum mechanics and the structure of the atom.[2]Atoms consist of a heavy central core called the nucleus surrounded by clouds of lightweight particles (electrons), called electron shells. The energy locked in the nucleus is enormous, but fortunately it cannot easily be released. The phenomenon we know as heat is simply the jiggling around of atoms and their components, so in principle a high enough temperature could cause the components of the core to break out. However, the temperature required to do this is in in the millions of degrees, so this cannot be achieved by any natural process that we know about. The second way that a nucleus could be disrupted is by particles striking it. However, the nucleus has a strong positive charge and the electron shells have a string negative charge. Any incoming negative charge would be deflected by the electron shell and any positive charge that penetrated the electron shells would be deflected by positive charge of the nucleus.
Outside influences
It is important that the sample not have had any outside influences. One example of this can be found in metamorphic rocks.[3] This does not mean that all rock samples are unreliable, but it is possible to account for a process which throws off the data for metamorphic rocks.
For example, with Uranium-lead dating with the crystallization of magma, this remains a closed system until the uranium decays. As it decays, it disrupts the crystal and allows the lead atom to move. Likewise, heating the rock such as granite forms gneiss or basalt forms schist. This can also disrupt the ratios of lead and uranium in the sample.
Dating metamorphic rocks has lead to incorrect dating of the Grand Canyon by the RATE Project (Radioisotopes and the Age of the Earth).[4][5]
Some major methods of radiometric dating
There are several major types of radiometric dating in use:[6],[7]
- Radiocarbon dating, also called carbon dating
- Potassium-argon dating
- Uranium-lead dating
- Uranium-thorium
- Rubidium-strontium dating
References
- ↑ Radiometric Time Scale USGS
- ↑ Principles of Quantum Mechanics page 89
- ↑ Radiometric Dating Course notes for EENS 211 at Tulane University
- ↑ Radioisotope Dating of Grand Canyon Rocks: Another Devastating Failure for Long-Age Geology (Note: be sure to make note of the first sentence of the second paragraph)
- ↑ Critique of RATE's methods Answers in Creation
- ↑ Quarternary Dating Methods, by M. Walker (Wiley & Sons, 2005).
- ↑ Isotopes: Principles and Applications, by G. Faure and T. Mensing (Wiley & Sons, 2005).