First Law of Thermodynamics
From Conservapedia
This is an old revision of this page, as edited by Tsumetai (Talk | contribs) at 14:58, March 15, 2007. It may differ significantly from current revision.
The First Law of Thermodynamics is that the increase in internal energy of a closed system equals the amount of heat energy added minus the work performed by the system.
Mathematically, this is described as follows:
where  is the infinitesimal increase in the internal energy,
is the infinitesimal increase in the internal energy,  is the infinitesimal amount of heat added, and
is the infinitesimal amount of heat added, and 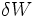 is the infinitesimal amount of work performed.
is the infinitesimal amount of work performed.
Note that if no energy is added, then the maximum amount of work that can be performed by the system is equal to its initial energy. This prevents the existence of a type of perpetual motion machine.
