Difference between revisions of "Carbon dating"
(→Known Limits in Carbon Dating: fact tag in place of text "citation?") |
|||
| Line 18: | Line 18: | ||
Since the half-life of <SUP>14</SUP>C is only 5715 years, after 50,000 years only about 1/500th of the <SUP>14</SUP>C remains - and since even initially it is only present as a minute proportion of the whole (0.0000000001% of all Carbon atoms), measuring the exact quantity present with precision enough to be of use for dating purposes is extremely difficult. For this reason, scientists do not generally attempt to carbon date material that is believed to be older than about 50,000-60,000 years old. However, isotopes with longer half-lives may be used. | Since the half-life of <SUP>14</SUP>C is only 5715 years, after 50,000 years only about 1/500th of the <SUP>14</SUP>C remains - and since even initially it is only present as a minute proportion of the whole (0.0000000001% of all Carbon atoms), measuring the exact quantity present with precision enough to be of use for dating purposes is extremely difficult. For this reason, scientists do not generally attempt to carbon date material that is believed to be older than about 50,000-60,000 years old. However, isotopes with longer half-lives may be used. | ||
| − | Additional anomalous results from carbon dating, which reinforce its limitations, include the inability to date carbonate rocks, which by confound the science behind the dating technique. | + | Additional anomalous results from carbon dating, which reinforce its limitations, include the inability to date carbonate rocks, which by confound the science behind the dating technique.{{fact}} |
== References == | == References == | ||
Revision as of 18:28, May 14, 2007
Carbon dating, or carbon-14 dating, is a method for comparing the ages of organic materials such as bones or things made from anything that once lived.
The technique is based on comparing the levels of 14C and 12C isotopes in the sample. When the sample was living, the ratio of 14C to 12C in the material is the same as in the atmosphere. But after death the 14C isotope decays into N-14, while 12C does not. The loss in 14C is then used to estimate how long ago the sample died, which is typically the same as how long the sample existed.
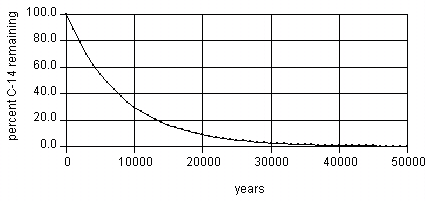
The rate of decay of 14C is such that 50% of the 14C in the sample will decay in 5730 years: 50% of the 14C in the sample is left, after 11460 years 25% will be left, after 17190 years 12.5% will be left, after 50,000 years only about 1/500th of the 14C remains - and since even initially it is only present as a minute proportion of the whole (0.0000000001% of all Carbon atoms)[1], measuring the exact quantity present accurately enough to be of use for dating purposes is extremely difficult. For this reason, scientists do not generally attempt to carbon date material that is believed to be older than about 50,000-60,000 years old.
Limits of Carbon Dating
First, it had not been proven scientifically whether the rate of decay of 14C has remained constant over hundreds or thousands of years: nor has it been proven that it has not remained constant. (Notably, the decay of other isotopes that have half-lives over the course of days to microseconds all follow the characteristic first-order kinetics; hundereds of isotopes follow this pattern, and their properties extenstively have been studied.) Some scientists have suggested, based on experimental observations, that the laws of physics do change over time.[2] The "change" discussed here - in a study that is still very controversial - occurred over billions of years, though, and not in the near enough future to have had an effect on the utility of carbon dating (since carbon dating is only useful to date life within the past 50,000 years, only a change within 50,000 years would affect the dating method).
Second, "various plants have differing abilities to exclude significant proportions of the C-14 in their intake. This varies with environmental conditions as well. The varying rates at which 14C is excluded in plants also means that the apparent age of a living animal may be affected by an animal's diet. An animal that ingested plants with relatively low 14C proportions would be dated older than their true age."[3]
Known Limits in Carbon Dating
Carbon dating cannot give accurate numbers if the sample is younger than 150 years or older than 50,000 years. The inaccuracy for material younger than 150 years is ascribed to changes in the ratio of 14C to 12C in the atmosphere.[4]
Since the half-life of 14C is only 5715 years, after 50,000 years only about 1/500th of the 14C remains - and since even initially it is only present as a minute proportion of the whole (0.0000000001% of all Carbon atoms), measuring the exact quantity present with precision enough to be of use for dating purposes is extremely difficult. For this reason, scientists do not generally attempt to carbon date material that is believed to be older than about 50,000-60,000 years old. However, isotopes with longer half-lives may be used.
Additional anomalous results from carbon dating, which reinforce its limitations, include the inability to date carbonate rocks, which by confound the science behind the dating technique.[Citation Needed]
References
- ↑ http://www.enviroliteracy.org/article.php/1006.html
- ↑ "The idea that nature's laws change over time was proposed in the 1930s by one of the titans in the history of physics, Paul Dirac of England. According to Dirac's large numbers hypothesis, the force of gravity changed over time." See Keay Davidson, "Recent study forces scientists to rethink basic law of physics," San Francisco Chronicle[1]
- ↑ Nondestructive Testing (NDT) Resource Center, "Uncertainty in Carbon Dating"[2]
- ↑ http://www.talkorigins.org/indexcc/CD/CD011.html