Heisenberg Uncertainty Principle
From Conservapedia
This is an old revision of this page, as edited by Jaques (Talk | contribs) at 08:15, March 27, 2007. It may differ significantly from current revision.
The Heisenberg Uncertainty Principle is a fundamental states that for any given quantum particle with position  and momentum
and momentum  , then the product of the standard deviations of the measurements of
, then the product of the standard deviations of the measurements of  and
and  must be of the order of Planck's constant (
must be of the order of Planck's constant ( ), which is a non-zero, positive-valued, real number.
), which is a non-zero, positive-valued, real number.
Mathematically it is represented with this equation: 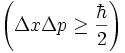 .
.
In English, this means - for example - that if we measure the position of the particle perfectly then there has to be a non-zero error in our measurement of momentum (and similarly the reverse) and so we can never know the exact position and momentum of a particle at any instant.