Carbon dating
Carbon dating, or carbon-14 dating, is a method for comparing the ages of organic materials such as bones or artifacts made from anything that once lived. Unlike many other radiometric dating methods, carbon dating has been calibrated for historical periods and within that range can give reliable results.
Contents
Principles
The technique is based on comparing the levels of 14C and 12C isotopes in the sample. 14C is produced in the atmosphere by cosmic ray neutrons replacing a proton in nitrogen (14N), producing 14C.[1]
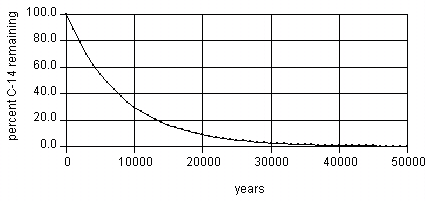
14C is unstable and decays back to 14N, at the rate of 50% every 5,730 years (so after 11,460 years 25% will be left, after 17,190 years 12.5% will be left, and so on).
In the meantime, however, the 14C will combine with oxygen in the atmosphere to form carbon dioxide, which enters the food chain via photosynthesis in plants.[1] By this means, most living things also have 14C and 12C in the same ratio as in the atmosphere. This ratio is about one 14C atom for every 1,000,000,000,000 12C atoms.[1]
However, when the sample dies, it stops ingesting 14C, so as the 14C decays to 14N, the ratio of 14C and 12C changes. This ratio of 14C to 12C is measured and a calculation turns the measurement into a figure representing how long ago the sample died.
Depending on the method used to measure the 14C, after about 50,000 years or so there is not enough left to measure, although advanced techniques can possibly stretch that to 100,000 years.[2] This then becomes the maximum age that can theoretically be derived by this method.
Limits of Carbon Dating
Carbon dating, like other radiometric dating methods, requires certain assumptions that cannot be scientifically proved. These include the starting conditions, the constancy of the rate of decay, and that no material has left or entered the sample.
Calibration
Unlike other radiometric dating techniques where it is not possible to calibrate the method against historically-known dates, limited calibration is possible for carbon dating. That is, samples with dates known from historical records can be used to check the accuracy of the method. Despite this, however, caution is still necessary in accepting dates derived from carbon dating.
Claims have been made of the method being calibrated back to 10,000 years using dendrochronology,[1] however these older dates derived via dendrochronology have themselves been derived by using carbon dating[3], making this circular reasoning.
Variable intake
Not all living things do have 14C:12C ratios the same as the atmosphere.
...various plants have differing abilities to exclude significant proportions of the C-14 in their intake. This varies with environmental conditions as well. The varying rates at which C-14 is excluded in plants also means that the apparent age of a living animal may be affected by an animal's diet. An animal that ingested plants with relatively low C-14 proportions would be dated older than their true age."[4]
Atmospheric variability
The method relies on the assumption that we know how much 14C is in the atmosphere, but this has been known to change. Nuclear testing since the 1950s has resulted in a large increase in the amount of 14C in the atmosphere, but because the levels have been measured since the 1950s, calculations can be adjusted for these changing levels, meaning that dating of recent samples is possible.[5]
However, atmospheric levels are also known to have changed since the start of the industrial revolution, making dating items from this period more difficult.[5]
Dating laboratories do not make any allowance for the change in atmospheric levels that would have occurred as a result of Noah's Flood. This means that radio-carbon dates cannot be used to prove that the Flood did not occur, because it assumes that it did not occur.[6]
See also
References
- ↑ 1.0 1.1 1.2 1.3 Higham, Thomas, Introduction, Radiocarbon web-info.
- ↑ Nave, R., Carbon Dating, Georgia State University.
- ↑ Batten, Don, Tree ring dating (dendrochronology) (Creation Ministries International).
- ↑ Nondestructive Testing (NDT) Resource Center, Carbon-14 Dating"
- ↑ 5.0 5.1 Higham, Thomas? K-12 Radiocarbon web-info.
- ↑ Batten, Don, (Ed.) What about carbon dating? Chapter 4 of the Creation Answers Book.