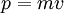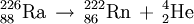|
|
| (316 intermediate revisions by 64 users not shown) |
| Line 1: |
Line 1: |
| − | {{Redirect|E{{=}}MC2}}
| + | '''E=mc²''' asserts that the energy ('''E''') in an unmoving particle is equal to the square of the [[speed of light]] ('''c²''') times the [[mass]] ('''m''') of that particle.<ref>"Energy and mass are linked in the most famous relationship in physics: E = mc². (The energy content of a body is equal to the mass of the body times the speed of light squared.)" [https://www.pbs.org/wgbh/nova/physics/einstein-genius-among-geniuses.html Einstein: Genius Among Geniuses] - PBS's NOVA</ref> The complete form, when applied to moving objects, is [[E^2=(mc^2)^2+(pc)^2|E²=(mc²)²+(pc)²]], where '''p''' represents momentum,<ref>https://www.youtube.com/watch?v=NnMIhxWRGNw</ref> It is a statement that purports to relate all [[matter]] to [[energy]]. In fact, no [[theory]] has successfully unified the [[law]]s governing [[mass]] (''i.e.'', [[gravity]]) with the laws governing light (''i.e.'', [[electromagnetism]]), and numerous attempts to derive ''E=mc²'' from first principles have failed.<ref name="wvarticles">Five lectures at Wikiversity. The 4th one derives the formula, using the assumptions in the "What the Equation Means" section. |
| − | [[File:Relativity3 Walk of Ideas Berlin.JPG|400px|right|thumb|4-meter-tall sculpture of [[Albert Einstein|Einstein]]'s 1905 ''E'' = ''mc''<sup>2</sup> formula at the 2006 [[Walk of Ideas]], [[Berlin]], [[Germany]]]]
| + | *[https://en.wikiversity.org/wiki/Special_relativity/space,_time,_and_the_Lorentz_transform Lecture 1] |
| − | {{special relativity}}
| + | *[https://en.wikiversity.org/wiki/Special_relativity/momentum Lecture 2] |
| − | [[File:E=mc2-explication.jpg|thumb|Explication]]
| + | *[https://en.wikiversity.org/wiki/Special_relativity/energy Lecture 3] |
| − | In [[physics]], in particular [[special relativity|special]] and [[general relativity]], '''mass–energy equivalence''' is the concept that the [[mass]] of a body is a measure of its [[energy]] content. In this concept, mass is a property of all energy; energy is a property of all mass; and the two properties are connected by a constant. This means (for example) that the total internal energy ''E'' of a body at rest is equal to the product of its [[Invariant mass|rest mass]] ''m'' and a suitable [[conversion factor]] to transform from [[:Category:Units of mass|units of mass]] to [[:Category:Units of energy|units of energy]]. [[Albert Einstein]] proposed mass–energy equivalence in 1905 in one of his [[Annus Mirabilis papers]] entitled "Does the inertia of a body depend upon its energy-content?"<ref name="inertia">{{Citation | author=Einstein, A. | year=1905 | title=Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig? | journal=Annalen der Physik | volume=18 | pages=639–643 | doi=10.1002/andp.19053231314|bibcode = 1905AnP...323..639E }}. See also the [http://www.fourmilab.ch/etexts/einstein/E_mc2/www/ English translation.]</ref> The equivalence is described by the famous equation:
| + | *[https://en.wikiversity.org/wiki/Special_relativity/E_%3D_mc%C2%B2 Lecture 4] |
| − | :<math>E = mc^2 \,\!</math>
| + | *[https://en.wikiversity.org/wiki/Special_relativity/spacetime_diagrams_and_vectors Lecture 5]</ref> [[Politics|Political]] pressure, however, has since made it impossible for anyone pursuing an academic career in [[science]] to even question the validity of this nonsensical [[equation]]. '''''Simply put, E=mc² is [[liberal claptrap]]'''''. |
| − | where ''E'' is energy, ''m'' is mass, and ''c'' is the [[speed of light]]. The formula is [[dimensional analysis|dimensionally consistent]] and does not depend on any specific [[systems of measurement|system of measurement units]]. | + | |
| − | The equation ''E'' = ''mc''<sup>2</sup> indicates that energy always exhibits [[relativistic mass]] in whatever form the energy takes.<ref name="tipler">{{Citation
| + | |
| − | |title=Modern Physics
| + | |
| − | |author=Paul Allen Tipler, Ralph A. Llewellyn
| + | |
| − | |pages=87–88
| + | |
| − | |url=http://books.google.com/?id=tpU18JqcSNkC&lpg=PP1&pg=PA87#v=onepage&q=
| + | |
| − | |isbn=0-7167-4345-0
| + | |
| − | |publisher=W. H. Freeman and Company
| + | |
| − | |date=2003-01
| + | |
| − | }}</ref> Mass–energy equivalence does not imply that mass may be "converted" to energy, but it allows for ''[[matter]]'' to be converted to energy. ''Mass'' remains conserved (i.e., the quantity of mass remains constant), since it is a property of matter and also any type of energy. Energy is also conserved. In physics, mass must be differentiated from matter. Matter, when seen as certain types of particles, ''can'' be created and destroyed (as in particle [[annihilation]] or [[Matter creation|creation]]), but a closed system of precursors and products of such reactions, as a whole, retain both the original mass and energy throughout the reaction.
| + | |
| | | | |
| − | When the system is not closed, and energy is ''removed'' from a system (for example in [[nuclear fission]] or [[nuclear fusion]]), some mass is always removed along with the energy, according to their equivalence where one always accompanies the other. This energy thus is associated with the missing mass, and this mass will be added to any other system which absorbs the removed energy. In this situation ''E'' = ''mc''<sup>2</sup> can be used to calculate how much mass goes along with the removed energy. It also tells how much mass will be added to any system which later absorbs this energy. This was the original use of the equation when derived by Einstein.
| + | The formula asserts that the mass of an object, at constant energy, magically varies precisely in inverse proportion to the square of a change in the speed of light over time,<ref>http://www.livescience.com/29111-speed-of-light-not-constant.html</ref> which violates [[conservation of mass]] and disagrees with commonsense.<ref>The formula asserts that the mass of an object has energy associated with it, even when it is not moving (p=0). The formula asserts a relationship between the rest mass of an object, its energy and the speed of light. According to the formula, the apparent mass of an object depends on its energy and so [[conservation of mass]] is not satisfied. Instead, relativity proposes that the total energy of a [[closed system]] is conserved, when we "convert" the masses into energies using this formula.</ref> |
| | | | |
| − | ''E'' = ''mc''<sup>2</sup> has sometimes been used as an explanation for the origin of energy in nuclear processes, but mass–energy equivalence does not explain the origin of such energies. Instead, this relationship merely indicates that the large amounts of energy released in such reactions may exhibit enough mass that the mass-loss may be measured, when the released energy (and its mass) have been removed from the system. For example, the loss of mass to an atom and a neutron as a result of the capture of the neutron, and the production of a gamma ray, has been used to test mass-energy equivalence to high precision, as the energy of the gamma ray may be compared with the mass defect after capture. In 2005, these were found to agree to 0.0004%, the most precise test of the equivalence of mass and energy to date. This test was performed in the [[World Year of Physics 2005]], a centennial celebration of Einstein's achievements in 1905.<ref name="rainville">Rainville, S. et al. World Year of Physics: A direct test of E=mc2. ''Nature'' 438, 1096-1097 (22 December 2005) | {{doi|10.1038/4381096a}}; Published online 21 December 2005.</ref>
| + | Physicists have never been able to unify light with matter<ref>Quantum Electrodynamics describes how matter interacts with matter, the standard model of particle physics describes how matter (fermions) interact with bosons (force carriers) for the electromagnetic, strong and weak forces. To date, no theory has been proven to unify gravity with electromagnetism.</ref> despite more than a billion-dollars-worth of attempts, and it is likely impossible to ever do so.<ref>Much of 20th century physics has centered around the interactions between photons (light) and fermionic matter, and much more than a billion dollars has been spent on this. But that doesn't imply that they have been "unified".</ref> [[Biblical Scientific Foreknowledge]] predicts that there is no unified theory of light and matter because they were created at different times, in different ways, as described in the [[Book of Genesis]]. |
| | | | |
| − | Einstein was not the first to propose a mass–energy relationship (see the [[#History|History]] section). However, Einstein was the first scientist to propose the ''E'' = ''mc''<sup>2</sup> formula and the first to interpret mass–energy equivalence as a fundamental principle that follows from the [[Spacetime symmetries|relativistic symmetries]] of [[Spacetime|space and time]].
| + | [[Mass]] is a measure of an object's inertia, in other words its resistance to acceleration. In contrast, the intrinsic [[energy]] of an object (such as an [[atom]]) is a function of electrostatic charge and other non-inertial forces, having nothing to do with gravity. Declaring the object's energy to be a function of inertia rather than electrostatics is an absurd and impossible attempt to unify the forces of nature, contrary to the accepted view (as predicted by [[Biblical Scientific Foreknowledge]]) that the forces of nature have not been unified. Liberal scientists assert the formula E=mc² is not limited to nuclear reactions; it applies to chemical reactions and even to the energy stored in a compressed spring.<ref>http://www.newton.dep.anl.gov/askasci/phy99/phy99140.htm</ref> |
| | | | |
| − | ==Nomenclature== | + | The claim that '''E=mc²''' has never yielded anything of value and it has often been used as a redefinition of "[[energy]]" for pseudo-scientific purposes by non-scientific journals. Claims can be found not only on liberal, second-tier college websites but at those of [[Baylor]] and the [[MIT]] that the equation is used in [[nuclear power]] generation and [[nuclear weapon]]s ([[nuclear fusion]] and [[nuclear fission]]) and about [[antimatter]].<ref>[http://www.pitt.edu/~jdnorton/teaching/HPS_0410/chapters/E=mcsquared/index.html John D. Norton ''Einstein for everyone - E=mc²''], Department of History and Philosophy of Science University of Pittsburgh</ref><ref>[http://hyperphysics.phy-astr.gsu.edu/hbase/relativ/releng.html Rod Nave ''HpyerPhysics - Relativistic Energy''], Georgia State University</ref><ref>[https://www.pbs.org/wgbh/nova/physics/legacy-of-e-equals-mc2.html Peter Tyson ''The Legacy of E=mc²''] October 11, 2005. PBS ''NOVA''.</ref> |
| | | | |
| − | In ''Does the inertia of a body depend upon its energy-content?'' Einstein used V to mean the [[speed of light]] in a vacuum and L to mean the [[energy]] lost by a body in the form of [[radiation]]. Consequently, the equation ''E'' = ''mc''<sup>2</sup> was not originally written as a formula but as a sentence in German that meant ''if a body gives off the energy L in the form of radiation, its mass diminishes by L/V<sup>2</sup>''.<ref>See the sentence on the last page (p.641) of the original edition of ''Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig?'' in Annalen der Physik, 1905, '''below''' the equation K<sub>0</sub> - K<sub>1</sub>= L/V<sup>2</sup> v<sup>2</sup>/2. See also the sentence '''under''' the last equation in the English translation, K<sub>0</sub> - K<sub>1</sub>= 1/2 L/c<sup>2</sup> v<sup>2</sup>, and the comment on the symbols used in ''About this edition'' that follows the translation [http://www.fourmilab.ch/etexts/einstein/E_mc2/www/]</ref> A remark placed above it informed that the equation was approximate because the conclusion was only justified if one neglected "magnitudes of fourth and higher orders" of a [[Series (mathematics)|series expansion]].<ref>See the sentence on the last page (p.641) of the original edition of ''Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig?'' in Annalen der Physik, 1905, '''above''' the equation K<sub>0</sub> - K<sub>1</sub>= L/V<sup>2</sup> v<sup>2</sup>/2. See also the sentence '''above''' the last equation in the English translation, K<sub>0</sub> - K<sub>1</sub>= 1/2 L/c<sup>2</sup> v<sup>2</sup>, and the comment on the symbols used in ''About this edition'' that follows the translation [http://www.fourmilab.ch/etexts/einstein/E_mc2/www/]</ref> In 1907, Einstein's mass-energy relationship was written as ''M<sub>0</sub>'' = ''E<sub>0</sub>/c''<sup>2</sup> by [[Max Planck]]<ref>{{cite journal |first=M. |last=Planck |journal=Ber.d.Berl.Akad. |volume=29 |issue= |pages=542 |year=1907 }}</ref> and, subsequently, was given a quantum interpretation<ref>{{cite journal |first=J. |last=Stark |title=Elementarquantum der Energie, Modell der negativen und der positiven Elekrizitat |journal=Physikalische Zeitschrift |page=881 |volume=24 |issue=8 |year=1907 }}</ref> by [[Johannes Stark]], who assumed its validity and correctness (''Gültigkeit''). However, Stark wrote the equation as e<sub>0</sub>=m<sub>0</sub> c<sup>2</sup> which meant the energy bound in the mass of an electron at rest and still was not the present popular version of the equation. In 1924, [[Louis de Broglie]] assumed the correctness of the relationship
| + | The [[Theory of Relativity]] has never been able to mathematically derive '''E=mc²''' from first principles,<ref name="wvarticles"/> and a physicist observed in a peer-reviewed paper published in 2011 that "Leaving aside that it continues to be affirmed experimentally, a rigorous proof of the mass-energy equivalence is probably beyond the purview of the special theory."<ref>[http://adsabs.harvard.edu/abs/2011AmJPh..79..591H Eugene Hecht: ''How Einstein confirmed E<sub>0</sub>=mc²'', American Journal of Physics, Volume 79, Issue 6, pp. 591-600 (2011)]</ref> Nevertheless, Robert Dicke - one of the most accomplished American-born physicists and experimental physicists in history - found it unlikely that the equivalence was wrong.<ref>R. H. Dicke "The Theoretical Significance of Experimental Relativity", Gordon and Breach, 1964</ref> |
| − | "énergie=masse c<sup>2</sup>" on page 31 in his ''Research on the Theory of the Quanta'' (published in 1925) but he did not write ''E'' = ''mc''<sup>2</sup>. However, Einstein returned to the topic once again after the World War Two and this time he wrote ''E'' = ''mc''<sup>2</sup> in the title of his article<ref>A.Einstein ''''E'' = ''mc''<sup>2</sup> '''': the most urgent problem of our time'' Science illustrated, vol. 1 no. 1, April issue, pp. 16-17, 1946 (item 417 in the "Bibliography"</ref> intended as an explanation for a general reader by analogy<ref>M.C.Shields ''Bibliography of the Writings of Albert Einstein to May 1951'' in Albert Einstein: Philosopher-Scientist by Paul Arthur Schilpp (Editor) [http://www.questia.com/PM.qst?a=o&d=84000079 Albert Einstein Philospher - Scientist]</ref>
| + | |
| | | | |
| − | ==Conservation of mass and energy== | + | It has been known for a long time that radiation has a mass equivalence, which was correctly derived by [[Henri Poincaré]] in 1904,<ref>[http://www.opticsinfobase.org/josa/abstract.cfm?uri=josa-42-8-540 Herbert E. Ives ''Derivation of the Mass-Energy Relation'', JOSA, Vol. 42, Issue 8, pp. 540-543 (1952)]</ref> but the equation '''E=mc²''' makes a claim far beyond that limited circumstance: |
| | | | |
| − | The concept of mass–energy equivalence connects the concepts of [[conservation of mass]] and [[conservation of energy]], which continue to hold separately in any [[isolated system]] (one that is closed to loss of any type of energy, including energy associated with loss of matter). The theory of relativity allows particles which have [[rest mass]] to be converted to other forms of mass which require motion, such as [[kinetic energy]], heat, or light. However, the system mass remains. Kinetic energy or light can also be converted to new kinds of particles which have rest [[mass]], but again the energy remains. Both the total mass and the total energy inside an isolated system remain constant over time, as seen by any single observer in a given inertial frame. | + | {{cquote|The equality of the mass equivalent of radiation to the mass lost by a radiating body is derivable from Poincaré’s momentum of radiation (1900) and his principle of relativity (1904).|||[[Herbert Ives]], 1952}} |
| | + | <!--In 1907, [[Max Planck]] proved in his fundamental paper that the formula ''E=mc²'' is not a general law and any system submitted to an external pressure will obey a different law: its mass will be proportional to its enthalpy ''H=E+PV'', that is, ''m=H/c²''<ref name="Capria">{{cite book |author=Marco M. Capria, Aubert Daigneaut et al. |title=Physics Before and After Einstein |publisher=IOS Press |year=2005 |chapter=2.Mechanics and Electromagnetism... |pages=43|isbn=1-58603-462-6 |url=http://www.dmi.unipg.it/~mamone/pubb/PBAE.pdf |quote=}}</ref>.--> |
| | | | |
| − | In other words, energy can neither be created nor destroyed, and energy, in all of its forms, has mass. Mass also can neither be created nor destroyed, and in all of its forms, has energy. According to the theory of relativity, mass and energy as commonly understood, are two names for the same thing, and neither one is changed nor transformed ''into'' the other. Rather, neither one exists without the other existing also, as a property of a system. Rather than mass being changed ''into'' energy, the view of [[special relativity]] is that rest mass has been changed to a more mobile form of mass, but remains mass. In the transformation process, neither the amount of mass nor the amount of energy changes, since both are properties which are connected to each other via a simple constant.<ref>In F. Fernflores. The Equivalence of Mass and Energy. Stanford Encyclopedia of Philosophy. [http://plato.stanford.edu/entries/equivME/#2.1]</ref> Thus, if energy leaves a system by changing its form, it simply takes its system mass with it. This view requires that if either mass or energy disappears from a system, it will always be found that both have simply moved off to another place, where they may both be measured as an increase of both mass and energy corresponding to the loss in the first system.
| + | == Description for the layman == |
| | | | |
| − | ===Fast-moving objects and systems of objects=== | + | The equation is extremely famous, and just as extremely misunderstood, in popular culture. Among the more outlandish claims are statements to the effect that "E=mc² holds the secret of the atomic bomb."<ref>Not so. The energy of the atomic bomb comes not from E=mc², but from the tension between the electrostatic force and the strong nuclear force. E=mc² simply meant that the fission products from the [[Little Boy|Hiroshima]] bomb weighed 0.7 grams less than the original Uranium.</ref> |
| | + | [[Image:600px-Albert Einstein Head.jpg|thumbnail|right|200px| |
| | + | *"I do not share the crusading spirit of the professional [[Atheism|atheist]] whose fervor is mostly due to a painful act of liberation from the fetters of religious indoctrination received in youth. I prefer an attitude of humility corresponding to the weakness of our intellectual understanding of nature and of our own being." - [[Albert Einstein]]<ref name="Isaacson390">Isaacson, Walter (2008). [https://books.google.com/books?id=cdxWNE7NY6QC&pg=PT390 ''Einstein: His Life and Universe''] (New York: Simon and Schuster), p. 390. Retrieved from GoogleBooks archive on February 19, 2015.</ref>]] |
| | + | The equation has acquired something of a "cult" status. In the USA, the popular ''[[Twilight Zone]]'' series featured '''E=mc²''' prominently, giving the equation greater currency with the public. The song ''[[Albert Einstein|Einstein]] A Go-Go'' by the band Landscape had a similar effect in the UK in the 1980s. The equation was the title of a single by ''Big Audio Dynamite'' in 1985, and an album by Mariah Carey in 2008. Some movies have been themed on this equation.<ref>https://www.imdb.com/title/tt0322120/?ref_=fn_tt_tt_2, https://www.imdb.com/title/tt0116160/?ref_=fn_tt_tt_1</ref> The equation, along with a picture of a mushroom cloud and a picture of [[Albert Einstein]], were featured on the front cover of an issue of ''Time'' magazine in 1946. All of this is disappointing when one considers how few people actually understand what the equation is saying. |
| | | | |
| − | When an object is pulled in the direction of motion, it gains momentum and energy, but when the object is already traveling near the [[speed of light]], it cannot move much faster, no matter how much energy it absorbs. Its momentum and energy continue to increase without bounds, whereas its speed approaches a constant value—the speed of light. This implies that in relativity the [[momentum]] of an object cannot be a constant times the [[velocity]], nor can the [[Kinetic energy#Kinetic energy of rigid bodies|kinetic energy]] be a constant times the square of the velocity.
| + | A number of science writers—both serious scientists and science popularizers—have at various times written their own explanation of the equation. Some of these are helpful; many are not. One of the better ones, though not without its share of nonsense, is a NOVA series by the [[Public Broadcasting Service]]<ref>[https://www.pbs.org/wgbh/nova/physics/ancestors-einstein.html David Bodanis ''Ancestors of E=mc²''], Nov 10, 2005, NOVA</ref> |
| | | | |
| − | A property called the [[relativistic mass]] is defined as the ratio of the momentum of an object to its velocity.<ref>Note that the relativistic mass, in contrast to the rest mass ''m<sub>0</sub>, is not a relativistic invariant, and that the velocity <math>\,v=dx^{(4)}/dt</math> is not a Minkowski four-vector, in contrast to the quantity <math>\tilde v=dx^{(4)}/d\tau</math>, where <math> d\tau =dt\cdot\sqrt{1-(v^2/c^2)}</math> is the differential of the [[proper time]]. However, the energy-momentum four-vector <math> p^{(4)}=m_0\cdot dx^{(4)}/d\tau</math> is a genuine Minkowski four-vector, and the intrinsic origin of the square-root in the definition of the relativistic mass is the distinction between '''dτ''' and '''dt'''.</ref> Relativistic mass depends on the motion of the object, so that different observers in relative motion see different values for it. If the object is moving slowly, the relativistic mass is nearly equal to the [[rest mass]] and both are nearly equal to the usual Newtonian mass. If the object is moving quickly, the relativistic mass is greater than the rest mass by an amount equal to the mass associated with the [[kinetic energy]] of the object. As the object approaches the speed of light, the relativistic mass grows infinitely, because the [[kinetic energy]] grows infinitely and this energy is associated with mass.
| + | In 2005 The PBS NOVA series also asked 10 physicists to describe the equation in layman's terms.<ref>[https://www.pbs.org/wgbh/nova/einstein/experts.html Lexi Krock, David Levin (editors) ''E=mc² explained'', June, 2005. PBS ''NOVA'']</ref> Here is a sample of five of the statements: |
| | + | {{cquote|''It's something that doesn't happen in your kitchen or in everyday life.''|||[[Neil deGrasse Tyson]], Astrophysicist, American Museum of Natural History}}{{cquote|''When an object emits light, say, a flashlight, it gets lighter.''|||Sheldon Glashow, Theoretical Physicist and Nobel Laureate, Boston University}}{{cquote|''Things that seem incredibly different can really be manifestations of the same underlying phenomena.''|||Nima Arkani-Hamed, Theoretical Physicist, Harvard University}}{{cquote|''You can get access to parts of nature you have never been able to get access to before.''|||Lene Hau, Experimental Physicist, Harvard University}}{{ |
| | + | cquote|''It certainly is not an equation that reveals all its subtlety in the few symbols that it takes to write down.''|||Brian Greene Theoretical Physicist Columbia University}} |
| | | | |
| − | The relativistic mass is always equal to the total energy (rest energy plus kinetic energy) divided by ''c''<sup>2</sup>.<ref name="tipler"/> Because the relativistic mass is exactly proportional to the energy, relativistic mass and relativistic energy are nearly synonyms; the only difference between them is the [[unit of measurement|units]]. If length and time are measured in [[natural units]], the speed of light is equal to 1, and even this difference disappears. Then mass and energy have the same units and are always equal, so it is redundant to speak about relativistic mass, because it is just another name for the energy. This is why physicists usually reserve the useful short word "mass" to mean rest-mass, or [[invariant mass]], and not relativistic mass.
| + | Of these, only the Sheldon Glashow quote makes a specific and meaningful statement about what the equation means. |
| | | | |
| − | The relativistic mass of a moving object is larger than the relativistic mass of an object that is not moving, because a moving object has extra kinetic energy. The ''rest mass'' of an object is defined as the mass of an object when it is at rest, so that the rest mass is always the same, independent of the motion of the observer: it is the same in all [[inertial frame]]s. | + | == What the Equation Means == |
| | + | The equation is about energy, both kinetic energy and potential energy. |
| | | | |
| − | For things and systems made up of many parts, like an [[atomic nucleus]], [[planet]], or [[star]], the relativistic mass is the sum of the relativistic masses (or energies) of the parts, because energies are additive in closed systems. This is not true in systems which are open, however, if energy is subtracted. For example, if a system is [[Binding_energy#Mass-energy_relation|''bound'']] by attractive forces, and the energy gained due to the forces of attraction in excess of the work done is removed from the system, then mass will be lost with this removed energy. For example, the mass of an atomic nucleus is less than the total mass of the protons and neutrons that make it up, but this is only true after this energy from binding has been removed in the form of a gamma ray (which in this system, carries away the mass of the energy of binding). This mass decrease is also equivalent to the energy required to break up the nucleus into individual protons and neutrons (in this case, work and mass would need to be supplied). Similarly, the mass of the solar system is slightly less than the masses of sun and planets individually.
| + | Kinetic energy is actual visible energy, that is, energy of things that are in motion. It's the energy of a thrown baseball. Radiation (for example, light) also counts as kinetic energy—it's the motion of photons. Light carries energy, force, and momentum. The force carried by light is not as obvious as the force of a thrown baseball, but it is there. The force of sunlight has been proposed for long-term space travel. It is also the force that causes the [[Pioneer anomaly]] and the force that makes a comet's tail stream away from the Sun. |
| | | | |
| − | For a system of particles going off in different directions, the [[invariant mass]] of the system is the analog of the rest mass, and is the same for all observers, even those in relative motion. It is defined as the total energy (divided by ''c''<sup>2</sup>) in the [[center of mass frame]] (where by definition, the system total momentum is zero). A simple example of an object with moving parts but zero total momentum, is a container of gas. In this case, the mass of the container is given by its total energy (including the kinetic energy of the gas molecules), since the system total energy and invariant mass are the same in any reference frame where the momentum is zero, and such a reference frame is also the only frame in which the object can be weighed. In a similar way, the theory of special relativity posits that the thermal energy in all objects (including solids) contributes to their total masses and weights, even though this energy is present as the kinetic and potential energies of the atoms in the object, and it (in a similar way to the gas) is not seen in the rest masses of the atoms that make up the object.
| + | Potential energy is the other kind—"hidden" energy. It can become kinetic energy, or vice-versa. A wound up spring, a charged battery, a stretched rubber band, a mixture of gasoline and air, an explosive, and a radioactive atom, all have potential energy. It's what is needed to make the principle of conservation of energy work. That is, when kinetic energy comes into existence, it's because potential energy was converted into kinetic energy. The wound-up spring of a clock has potential energy, that runs down over time, being converted into the kinetic energy of the ticking sound. A battery has potential energy that runs down when it provides electricity to make things move. Various chemical substances have characteristic amounts of potential energy, that may be converted to or from kinetic energy when chemical reactions occur. For example, Sodium and Chlorine have more potential energy than Sodium Chloride. Explosives have more potential energy than their constituent atoms. Radioactive atoms have more potential energy than their "daughter" atoms. |
| | | | |
| − | In a similar manner, even photons (light quanta), if trapped in a container space (as a [[photon gas]] or [[thermal radiation]]), would contribute a mass associated with their energy to the container. Such an extra mass, in theory, could be weighed in the same way as any other type of rest mass. This is true in special relativity theory, even though individually, photons have no rest mass. The property that trapped energy ''in any form'' adds weighable mass to systems that have no net momentum, is one of the characteristic and notable consequences of relativity. It has no counterpart in classical Newtonian physics, in which radiation, light, heat, and kinetic energy never exhibit weighable mass under any circumstances.
| + | The principle of ''conservation of energy'', universally accepted for well over 100 years, says |
| | | | |
| − | Just as the relativistic mass of closed system is conserved through time, so also is its invariant mass. It is this property which allows the conservation of all types of mass in systems, and also conservation of all types of mass in reactions where matter is converted to energy, and vice versa.
| + | ::Total energy (kinetic + potential) is always conserved. |
| | | | |
| − | ==Applicability of the strict mass–energy equivalence formula, ''E'' = ''mc''²==
| + | Hundreds of years of research by chemists (and, before that, the alchemists) worked out the potential energies that are characteristic of various substances, and that the potential and kinetic energies are accurately converted from one to the other, leading to the principle of conservation of total energy. |
| | | | |
| − | As is noted above, two different definitions of mass have been used in special relativity, and also two different definitions of energy. The simple equation ''E'' = ''mc''² is not generally applicable to all these types of mass and energy, except in the special case that the total additive momentum is zero for the system under consideration. In such a case, which is always guaranteed when observing the system from either its [[center of mass frame]] or its [[center of momentum frame]], ''E'' = ''mc''² is always true for any type of mass and energy that are chosen. Thus, for example, in the center of mass frame, the total energy of an object or system is equal to its rest mass times ''c''², a useful equality. This is the relationship used for the container of gas in the previous example. It is ''not'' true in other reference frames where the center of mass is in motion. In these systems or for such an object, its total energy will depend on both its rest (or invariant) mass, and also its (total) momentum.<ref>Relativity DeMystified, D. McMahon, Mc Graw Hill (USA), 2006, ISBN 0-07-145545-0</ref>
| + | An interesting fact is that, normally, one considers only ''changes'' in potential energy; one doesn't need an absolute scale. A rock at the top of a hill has more potential energy than after it rolls to the bottom of the hill, but the energy at the bottom isn't necessarily zero. We could dig a hole and let it roll down farther, with its energy going negative. Only changes matter. Now it turns out that, once one accepts the implications of E=mc², one ''could'' assign an absolute potential energy to something—its mass times c², and changes in potential energy would work out correctly because of the mass changes. But that isn't necessary, and, in any case, it would require accepting E=mc² and would therefore be getting ahead of the story. |
| | | | |
| − | In inertial reference frames other than the rest frame or center of mass frame, the equation ''E'' = ''mc''² remains true if the energy is the relativistic energy ''and'' the mass the relativistic mass. It is also correct if the energy is the rest or invariant energy (also the minimum energy), ''and'' the mass is the rest mass, or the invariant mass. However, connection of the '''total or relativistic energy''' ('''E<sub>r</sub>''') with the '''rest or invariant mass''' ('''m<sub>0</sub>''') requires consideration of the system total momentum, in systems and reference frames where the total momentum has a non-zero value. The formula then required to connect the two different kinds of mass and energy, is the extended version of Einstein's equation, called the [[Mass in special relativity#The relativistic energy-momentum equation|relativistic energy–momentum relationship]]:<ref>Dynamics and Relativity, J.R. Forshaw, A.G. Smith, Wiley, 2009, ISBN 978-0-470-01460-8</ref>
| |
| | | | |
| − | ::<math>E_r^2 - |\vec{p} \,|^2 c^2 = m_0^2 c^4</math> | + | With those preliminaries out of the way, it is possible to give a concise explanation of what the equation means: |
| | | | |
| − | ::<math>E_r^2 - (pc)^2 = (m_0 c^2)^2\,</math> | + | ::'''Potential energy has mass.''' |
| | | | |
| − | or | + | That is, it weighs something. Whenever anything has potential energy of any kind in it, improbable as this may sound, it weighs more. The proportionality constant is 1/c<sup>2</sup>, or 1.11 x 10<sup>−17</sup> kilograms per joule. A fresh battery weighs more than a spent one, a wound-up alarm clock weighs more than a run-down one, etc. |
| | | | |
| − | ::<math>E_r = \sqrt{ (m_0 c^2)^2 + (pc)^2 } \,\!</math>
| + | Now that's way too small to measure for anything other than nuclear reactions, which is why it escaped everyone's notice for so long. But it has been measured and experimentally verified for nuclear transformations all across the periodic table. |
| | | | |
| − | Here the ''(pc)<sup>2</sup>'' term represents the square of the [[Euclidean norm]] (total vector length) of the various momentum vectors in the system, which reduces to the square of the simple momentum magnitude, if only a single particle is considered. This equation reduces to ''E'' = ''mc''² when the momentum term is zero. For photons where '''m<sub>0</sub> = 0''', the equation reduces to '''E<sub>r</sub> = pc'''.
| + | :There's an interesting parallel with heat. Before the rise of thermodynamics, it was believed that heat was a "substance". That substance was called "caloric". When heat travels from one body to another, what was really happening was presumed to be a transfer of caloric. Much effort was put into measuring the mass of this mysterious "substance". It was always found to be zero, and we now know that what is actually being transferred is thermal energy. So it is not unheard-of to assign mass to intangible properties. |
| | | | |
| − | ''View a video explanation of the full equation at [http://www.youtube.com/watch?v=NnMIhxWRGNw Minute Physics on Youtube]''
| + | The nonzero mass of potential energy, and the equation E=mc², were determined on theoretical grounds, before any experimental observations were made. The logic of this follows from these assumptions: |
| | | | |
| − | ==Meanings of the strict mass–energy equivalence formula, ''E = mc''²==
| + | #Galilean and Newtonian mechanics. |
| | + | #Galilean relativity, that is, the notion that there is no absolute frame of reference. |
| | + | #Conservation of energy. |
| | + | #Conservation of momentum. (So far this is just classical physics.) |
| | + | #The universality of the speed of light. (That is, special relativity.) |
| | | | |
| − | [[File:E equals m plus c square at Taipei101.jpg|thumb|right|The mass–energy equivalence formula was displayed on [[Taipei 101]] during the event of the [[World Year of Physics 2005]].]]
| + | Keep in mind that, under special relativity, it's not just space and time that need to be redefined. The definitions of momentum and energy need to change also. This is necessary so that the '''conservation of energy and of momentum will be absolutely precise in all circumstances.''' |
| | | | |
| − | Mass–energy equivalence states that any object has a certain energy, even when it is stationary. In [[Newtonian mechanics]], a motionless body has no [[kinetic energy]], and it may or may not have other amounts of internal stored energy, like [[chemical energy]] or [[thermal energy]], in addition to any [[potential energy]] it may have from its position in a [[field (physics)|field of force]]. In Newtonian mechanics, all of these energies are much smaller than the mass of the object times the speed of light squared.
| + | Under classical Newtonian mechanics, the momentum and kinetic energy of a moving mass are |
| | + | :<math>p = mv\,</math> |
| | + | and |
| | + | :<math>E = \frac{1}{2}mv^2\,</math> |
| | + | respectively. But under special relativity they are |
| | + | :<math>p = \frac{mv}{\sqrt{1 - v^2/c^2}}\,</math> |
| | + | and |
| | + | :<math>E = mc^2\left(\frac{1}{1 - v^2/c^2} - 1\right)\,</math> |
| | + | One can verify that, in the non-relativistic limit, the relativistic values converge to the classical ones. |
| | | | |
| − | In relativity, all of the energy that moves along with an object (that is, all the energy which is present in the object's rest frame) contributes to the total mass of the body, which measures how much it resists acceleration. Each potential and kinetic energy makes a proportional contribution to the mass. As noted above, even if a box of ideal mirrors "contains" light, then the individually massless photons still contribute to the total mass of the box, by the amount of their energy divided by c<sup>2</sup>.<ref>{{cite book
| + | It is this requirement, and some "''gedanken experiments''"<ref>https://www.britannica.com/science/Gedankenexperiment</ref> involving conversion between potential and kinetic energy, that lead to E=mc².<ref name="wvarticles"/> These experiments involve some kind of object that isn't moving (though there might be internal motion that doesn't figure in the experiment) and therefore has no kinetic energy and only potential energy, turning into some things that have kinetic energy. The requirements of strict conservation of total momentum and total energy prove the equation. |
| − | |title=Mechanics
| + | |
| − | |edition=2
| + | |
| − | |first1=H. S.
| + | |
| − | |last1=Hans
| + | |
| − | |first2=S. P.
| + | |
| − | |last2=Puri
| + | |
| − | |publisher=Tata McGraw-Hill
| + | |
| − | |year=2003
| + | |
| − | |isbn=0-07-047360-9
| + | |
| − | |page=433
| + | |
| − | |url=http://books.google.com/books?id=hrBe52GPHrYC}}, [http://books.google.com/books?id=hrBe52GPHrYC&pg=PA433 Chapter 12 page 433]
| + | |
| − | </ref>
| + | |
| | | | |
| − | In [[Theory of relativity|relativity]], removing energy is removing mass, and for an observer in the center of mass frame, the formula ''m'' = ''E''/''c''² indicates how much mass is lost when energy is removed. In a nuclear reaction, the mass of the atoms that come out is less than the mass of the atoms that go in, and the difference in mass shows up as heat and light which has the same relativistic mass as the difference (and also the same [[invariant mass]] in the center of mass frame of the system). In this case, the ''E'' in the formula is the energy released and removed, and the mass ''m'' is how much the mass decreases. In the same way, when any sort of energy is added to an isolated system, the increase in the mass is equal to the added energy divided by ''c''². For example, when water is heated it gains about {{val|1.11|e=-17|u=kg}} of mass for every joule of heat added to the water.
| + | Einstein's famous derivation<ref name="einstein1905b">[http://www.fourmilab.ch/etexts/einstein/E_mc2/www/ "Does the Inertia of a Body Depend its Energy Content?" Albert Einstein, Sept 1905]</ref> involved light instead of tangible objects, but the result is the same. |
| | | | |
| − | An object moves with different speed in different frames, depending on the motion of the observer, so the kinetic energy in both Newtonian mechanics and relativity is ''frame dependent''. This means that the amount of relativistic energy, and therefore the amount of relativistic mass, that an object is measured to have depends on the observer. The ''rest mass'' is defined as the mass that an object has when it is not moving (or when an inertial frame is chosen such that it is not moving). The term also applies to the invariant mass of systems when the system as a whole is not "moving" (has no net momentum). The rest and invariant masses are the smallest possible value of the mass of the object or system. They also are conserved quantities, so long as the system is closed. Because of the way they are calculated, the effects of moving observers are subtracted, so these quantities do not change with the motion of the observer.
| + | ==History of Experimental Verification== |
| | + | Because the change in mass arising from a given release of energy is so small (<math>1/c^2</math>, which 1.11 x 10<sup>−17</sup> kilograms per joule), it is essentially impossible to check this equation for normal processes. For example, a flashlight battery loses about 1 picogram of mass when it discharges, and the resultant atoms from the detonation of 1 kilogram of TNT weigh 47 nanograms less than the TNT. Even if all the particles of smoke and gas could be collected reliably, the difference couldn't be detected. |
| | | | |
| − | The rest mass is almost never additive: the rest mass of an object is not the sum of the rest masses of its parts. The rest mass of an object is the total energy of all the parts, including kinetic energy, as measured by an observer that sees the center of the mass of the object to be standing still. The rest mass adds up only if the parts are standing still and do not attract or repel, so that they do not have any extra kinetic or potential energy. The other possibility is that they have a positive kinetic energy and a negative potential energy that exactly cancels.
| + | Measuring the effect requires process that release vastly more energy than ordinary chemical processes. The discovery of Radium and Polonium around 1898 gave a tantalizing hint that there were processes that released far more energy than chemical processes could account for. These elements continuously released measurable heat, and also glowed in the dark. |
| | | | |
| − | === Binding energy and the "mass defect" === | + | Einstein touched on this possibility in his original 1905 paper.<ref name=einstein1905b/> |
| − | {{main|binding energy}} | + | {{cquote|''It is not impossible that with bodies whose energy content is variable to a high degree (e.g. with radium salts) the theory may be successfully put to the test.''}}It would take more than a decade to develop an understanding of the nuclear process involved. The first thing that was required was accurate knowledge of atomic weights. |
| | | | |
| − | Whenever any type of energy is removed from a system, the mass associated with the energy is also removed, and the system therefore loses mass. This mass defect in the system may be simply calculated as '''Δm = ΔE/c<sup>2</sup>''', and this was the form of the equation historically first presented by Einstein in 1905. However, use of this formula in such circumstances has led to the false idea that mass has been "converted" to energy. This may be particularly the case when the energy (and mass) removed from the system is associated with the ''binding energy'' of the system. In such cases, the binding energy is observed as a "mass defect" or deficit in the new system. The fact that the released energy is not easily weighed in many such cases, may cause its mass to be neglected as though it no longer existed. This circumstance, along with the real conversion of matter (not mass) to energy in some high energy particle reactions, has caused the conversion of ''matter'' to energy (which occurs) to be conflated with the false idea of conversion of ''mass'' to energy, which does not occur.
| + | Atomic weights of the various elements were first measured, with accuracy of a few decimal places, by J. J. Berzelius in the late 1820s. This required extremely painstaking (for the time) measurements. The figures were refined to even more accuracy by J. A. R. Newlands in the 1860s. The values were accurate enough to clearly show the rather interesting property that the atomic weights were nearly integers, but not exactly so. The reason for this would turn out to be partly because of different isotopes (discovered by Frederick Soddy in 1913) and partly because of E=mc<sup>2</sup>. |
| | | | |
| − | The difference between the rest mass of a bound system and of the unbound parts is the [[binding energy]] of the system, if this energy has been removed after binding. For example, a water molecule weighs a little less than two free hydrogen atoms and an oxygen atom; the minuscule mass difference is the energy that is needed to split the molecule into three individual atoms (divided by ''c''²), and which was given off as heat when the molecule formed (this heat had mass). Likewise, a stick of dynamite in theory weighs a little bit more than the fragments after the explosion, but this is true only so long as the fragments are cooled and the heat removed. In this case the mass difference is the energy/heat that is released when the dynamite explodes, and when this heat escapes, the mass associated with it escapes, only to be deposited in the surroundings which absorb the heat (so that total mass is conserved).
| + | In 1907 Rutherford determined that the "alpha" radiation from Radium was Helium. In 1911 he formulated the theory of the nucleus. In 1919 he demonstrated that nuclear transmutations could take place, such as |
| | | | |
| − | Such a change in mass may only happen when the system is open, and the energy and mass escapes. Thus, if a stick of dynamite is blown up in a hermetically sealed chamber, the mass of the chamber and fragments, the heat, sound, and light would still be equal to the original mass of the chamber and dynamite. If sitting on a scale, the weight and mass would not change. This would in theory also happen even with a nuclear bomb, if it could be kept in an ideal box of infinite strength, which did not rupture or pass radiation.<ref>E. F. Taylor and J. A. Wheeler, '''Spacetime Physics''', W.H. Freeman and Co., NY. 1992. ISBN 0-7167-2327-1, see pp. 248-9 for discussion of mass remaining constant after detonation of nuclear bombs, until heat is allowed to escape.</ref> Thus, a 21.5 [[TNT equivalent|kiloton]] (9 x 10<sup>13</sup> joule) nuclear bomb produces about one gram of heat and electromagnetic radiation, but the mass of this energy would not be detectable in an exploded bomb in an ideal box sitting on a scale; instead, the contents of the box would be heated to millions of degrees without changing total mass and weight. If then, however, a transparent window (passing only electromagnetic radiation) were opened in such an ideal box after the explosion, and a beam of X-rays and other lower-energy light allowed to escape the box, it would eventually be found to weigh one gram less than it had before the explosion. This weight-loss and mass-loss would happen as the box was cooled by this process, to room temperature. However, any surrounding mass which had absorbed the X-rays (and other "heat") would '''gain''' this gram of mass from the resulting heating, so the mass "loss" would represent merely its relocation. Thus, no mass (or, in the case of a nuclear bomb, no matter) would be "converted" to energy in such a process. Mass and energy, as always, would both be separately conserved.
| + | ::<math>{}_7^{14}\mathrm{N}\, +\, {}_2^4\mathrm{He}\,\rightarrow\, {}_8^{17}\mathrm{O}\, +\, {}_1^1\mathrm{H}</math> |
| | | | |
| − | === Massless particles ===
| + | The discovery of Radon, and much further investigation, revealed that the behavior of Radium was |
| − | Massless particles have zero rest mass. Their relativistic mass is simply their relativistic energy, divided by c<sup>2</sup>, or m(relativistic) = E/c<sup>2</sup>.<ref>{{cite book
| + | |
| − | |title=Basic relativity
| + | |
| − | |edition=2
| + | |
| − | |first1=Richard A.
| + | |
| − | |last1=Mould
| + | |
| − | |publisher=Springer
| + | |
| − | |year=2002
| + | |
| − | |isbn=0-387-95210-1
| + | |
| − | |page=126
| + | |
| − | |url=http://books.google.com/books?id=lfGE-wyJYIUC}}, [http://books.google.com/books?id=lfGE-wyJYIUC&pg=PA126 Chapter 5 page 126]
| + | |
| − | </ref><ref>{{cite book
| + | |
| − | |title=Introduction to electromagnetic theory: a modern perspective
| + | |
| − | |first1=Tail L.
| + | |
| − | |last1=Chow
| + | |
| − | |publisher=Jones & Bartlett Learning
| + | |
| − | |year=2006
| + | |
| − | |isbn=0-7637-3827-1
| + | |
| − | |page=392
| + | |
| − | |url=http://books.google.com/books?id=dpnpMhw1zo8C}}, [http://books.google.com/books?id=dpnpMhw1zo8C&pg=PA392 Chapter 10 page 392]
| + | |
| − | </ref> The energy for photons is '''E = hf''' where '''h''' is [[Planck constant|Planck's constant]] and '''f''' is the photon frequency. This frequency and thus the relativistic energy are frame-dependent.
| + | |
| | | | |
| − | If an observer runs away from a photon in the direction it travels from a source, having it catch up with the observer, then when the photon catches up it will be seen as having less energy than it had at the source. The faster the observer is traveling with regard to the source when the photon catches up, the less energy the photon will have. As an observer approaches the speed of light with regard to the source, the photon looks redder and redder, by [[relativistic Doppler effect]] (the Doppler shift is the relativistic formula), and the energy of a very long-wavelength photon approaches zero. This is why a photon is ''massless''; this means that the rest mass of a photon is zero.
| + | ::<math>{}_{88}^{226}\mathrm{Ra}\,\rightarrow\, {}_{86}^{222}\mathrm{Rn}\, +\, {}_2^4\mathrm{He}</math> |
| | + | and |
| | + | ::<math>{}_{86}^{222}\mathrm{Rn}\,\rightarrow\, {}_{84}^{218}\mathrm{Po}\, +\, {}_2^4\mathrm{He}</math> |
| | | | |
| − | === Massless particles contribute rest mass and invariant mass to systems === | + | Accurate ways of measuring speed of a charged particle, by deflecting it in a magnetic field, had been developed by then, so that, by very painstaking observation and measurement, it was determined that the first alpha particle (Helium nucleus) had an energy of 4.78 MeV and the second an energy of 5.49 Mev. This confirmed E=mc<sup>2</sup> up to the accuracy of the measurements. The equation, along with knowledge of isotope mixes, now explained why the atomic weights appearing in the periodic table were nearly integers, but not exactly so. |
| | | | |
| − | Two photons moving in different directions cannot both be made to have arbitrarily small total energy by changing frames, or by moving toward or away from them. The reason is that in a two-photon system, the energy of one photon is decreased by chasing after it, but the energy of the other will increase with the same shift in observer motion. Two photons not moving in the same direction will exhibit an [[inertial frame]] where the combined energy is smallest, but not zero. This is called the [[center of mass]] frame or the [[center of momentum]] frame; these terms are almost synonyms (the center of mass frame is the special case of a center of momentum frame where the center of mass is put at the origin). The most that chasing a pair of photons can accomplish to decrease their energy is to put the observer in frame where the photons have equal energy and are moving directly away from each other. In this frame, the observer is now moving in the same direction and speed as the center of mass of the two photons. The total momentum of the photons is now zero, since their momentums are equal and opposite. In this frame the two photons, as a system, have a mass equal to their total energy divided by ''c''<sup>2</sup>. This mass is called the [[invariant mass]] of the pair of photons together. It is the smallest mass and energy the system may be seen to have, by any observer. It is only the invariant mass of a two-photon system that can be used to make a single particle with the same rest mass.
| + | Around 1925, the development of the mass spectrograph, by Francis Aston, made it possible to measure atomic weights to extreme precision. |
| | | | |
| − | If the photons are formed by the collision of a particle and an antiparticle, the invariant mass is the same as the total energy of the particle and antiparticle (their rest energy plus the kinetic energy), in the center of mass frame, where they will automatically be moving in equal and opposite directions (since they have equal momentum in this frame). If the photons are formed by the disintegration of a ''single'' particle with a well-defined rest mass, like the neutral [[pion]], the invariant mass of the photons is equal to rest mass of the pion. In this case, the center of mass frame for the pion is just the frame where the pion is at rest, and the center of mass does not change after it disintegrates into two photons. After the two photons are formed, their center of mass is still moving the same way the pion did, and their total energy in this frame adds up to the mass energy of the pion. Thus, by calculating the [[invariant mass]] of pairs of photons in a particle detector, pairs can be identified that were probably produced by pion disintegration.
| + | The 1932 Cockcroft-Walton experiment, described in more detail below, started to make the equation famous by confirming it, with reasonable accuracy, for an artificially induced nuclear reaction. (Confirming E=mc<sup>2</sup> was not a goal of the experiment; it was an incidental consequence. The equation had already been known and understood for many years.) |
| | | | |
| − | A similar calculation illustrates that the invariant mass of systems is conserved, even when massive particles (particles with rest mass) within the system are converted to massless particles (such as photons). In such cases, the photons contribute invariant mass to the system, even though they individually have no invariant mass or rest mass. Thus, an electron and positron (each of which has rest mass) may undergo [[electron-positron annihilation|annihilation]] with each other to produce two photons, each of which is massless (has no rest mass). However, in such circumstances, no system mass is lost. Instead, the system of both photons moving away from each other has an invariant mass, which acts like a rest mass for any system in which the photons are trapped, or that can be weighed. Thus, not only the quantity of relativistic mass, but also the quantity of invariant mass does not change in transformations between "matter" (electrons and positrons) and energy (photons).
| + | In the decades since, nuclear transmutations have been performed, in particle accelerators, all over the periodic table, observing in detail the properties of various isotopes. These have confirmed E=mc<sup>2</sup> with great precision. See [[Quantitative Analysis of Alpha Decay]]. |
| | | | |
| − | ===Relation to gravity=== | + | ==The Rainville test== |
| − | In physics, there are two distinct concepts of [[mass]]: the gravitational mass and the inertial mass. The gravitational mass is the quantity that determines the strength of the [[gravitational field]] generated by an object, as well as the gravitational force acting on the object when it is immersed in a gravitational field produced by other bodies. The inertial mass, on the other hand, quantifies how much an object accelerates if a given force is applied to it. The mass-energy equivalence in special relativity refers to the inertial mass. However, already in the context of Newton gravity, the Weak [[Equivalence Principle]] is postulated: the gravitational and the inertial mass of every object are the same. Thus, the mass-energy equivalence, combined with the Weak Equivalence Principle, results in the prediction that all forms of energy contribute to the gravitational field generated by an object. This observation is one of the pillars of the [[general theory of relativity]].
| + | Perhaps the most precise direct empirical verification of E=mc<sup>2</sup> was done in 2005 by Simon Rainville ''et. al.''<ref>[http://www.nature.com/nature/journal/v438/n7071/full/4381096a.html Nature 438, 1096-1097 (22 December 2005)] doi:10.1038/4381096a; Published online 21 December 2005</ref> The article states that "Einstein's relationship is separately confirmed in two tests, which yield a combined result of 1−Δmc²/E=(−1.4±4.4)×10<sup>−7</sup>, indicating that it holds to a level of at least 0.00004%. To our knowledge, this is the most precise direct test of the famous equation yet described." |
| | | | |
| − | The above prediction, that all forms of energy interact gravitationally, has been subject to experimental tests. The first observation testing this prediction was made in 1919.<ref>{{cite journal|last=Dyson|coauthors= Eddington, A.S., & Davidson, C.R. |year=1920 |title=A Determination of the Deflection of Light by the Sun's Gravitational Field, from Observations Made at the Solar eclipse of May 29, 1919|journal= [[Philosophical Transactions of the Royal Society A: Physical, Mathematical and Engineering Sciences|Phil. Trans. Roy. Soc. A]]|volume=220|issue=571-581|pages= 291–333|bibcode=1920RSPTA.220..291D|doi=10.1098/rsta.1920.0009|first =F.W.}}</ref> During a [[solar eclipse]], [[Arthur Eddington]] observed that the light from stars passing close to the Sun was bent. The effect is due to the gravitational attraction of light by the sun. The observation confirmed that the energy carried by light indeed is equivalent to a gravitational mass. Another seminal experiment, the [[Pound–Rebka experiment]], was performed in 1960.<ref>{{cite journal|last=Pound| first=R. V.| coauthors = Rebka Jr. G. A. | date= April 1, 1960| title=Apparent weight of photons| journal=[[Physical Review Letters]]| volume=4| issue=7| pages=337–341| doi = 10.1103/PhysRevLett.4.337| bibcode=1960PhRvL...4..337P}}</ref> In this test a beam of light was emitted from the top of a tower and detected at the bottom. The frequency of the light detected was higher than the light emitted. This result confirms that the energy of photons increases when they fall in the gravitational field of the earth. The energy, and therefore the gravitational mass, of photons is proportional to their frequency as stated by the [[Planck constant|Planck's relation]].
| + | ==The [[Cockcroft and Walton Experiment|Cockcroft/Walton experiment]]== |
| | + | This experiment is not one usually cited as validating E=mc². That was not its goal. The generally accepted important tests of this equation are the measurements of alpha decay energies, described above. |
| | | | |
| − | ==Consequences for nuclear physics==
| + | In 1932 English physicist John Cockcroft and Irish physicist Ernest Walton performed the first artificial nuclear transmutation of nuclei, for which they were awarded the 1951 [[Nobel Prize]] in physics.<ref>[https://www.nobelprize.org/nobel_prizes/physics/laureates/1951/cockcroft-lecture.pdf John D. Cockroft ''Experiments on the interaction of high-speed nucleons with atomic nuclei''], Nobel Lecture, Dec 11, 1951</ref> The award was for ''"their pioneer work on the transmutation of atomic nuclei by artificially accelerated atomic particles."''<ref>[https://www.nobelprize.org/nobel_prizes/physics/laureates/1951/# Nobel Prize Organization]</ref> |
| − | [[Image:TaskForce One.jpg|thumb|right|'''Task Force One''', the world's first nuclear-powered task force. {{USS|Enterprise|CVN-65|2}}, {{USS|Long Beach|CGN-9|2}} and {{USS|Bainbridge|CGN-25|2}} in formation in the Mediterranean, 18 June 1964. ''Enterprise'' crew members are spelling out Einstein's Mass-Energy Equivalence formula ''E''=''mc''² on the flight deck.]] | + | |
| | | | |
| − | [[Max Planck]] pointed out that the mass–energy equivalence formula implied that bound systems would have a mass less than the sum of their constituents, once the binding energy had been allowed to escape. However, Planck was thinking about chemical reactions, where the binding energy is too small to measure. Einstein suggested that radioactive materials such as [[radium]] would provide a test of the theory, but even though a large amount of energy is released per atom in radium, due to the [[half-life]] of the substance (1602 years), only a small fraction of radium atoms decay over an experimentally measurable period of time.
| + | Verifying E=mc² was not the goal of the experiment, and the Nobel prize was awarded for the transmutation itself, not any verification of the equation. This experiment could not have proved any general truth to the equation, since it was a test of just one specific reaction. But data from this experiment was consistent with the equation for the particular transmutation involved. |
| | | | |
| − | Once the nucleus was discovered, experimenters realized that the very high binding energies of the atomic nuclei should allow calculation of their binding energies, simply from mass differences. But it was not until the discovery of the [[neutron]] in 1932, and the measurement of the neutron mass, that this calculation could actually be performed (see [[nuclear binding energy]] for example calculation). A little while later, the first [[Nuclear transmutation|transmutation]] reactions (such as<ref>[http://homepage.eircom.net/~louiseboylan/Pages/Cockroft_walton.htm] Cockcroft-Walton experiment</ref> the [[Cockcroft-Walton experiment]]: <sup>7</sup>Li + ''p'' → 2 <sup>4</sup>He) verified Einstein's formula to an accuracy of ±0.5%.
| + | They bombarded [[Lithium]] atoms with [[protons]] having a [[kinetic energy]] less than 1 [[Electron-Volts|MeV]]. The result were two (slightly less heavy) [[alpha particle]]s, for which the [[kinetic energy]] was measured as 17.3 MeV |
| − | In 2005, Rainville et al. published a direct test of the energy-equivalence of mass lost in the binding-energy of a neutron to atoms of particular isotopes of silicon and sulfur, by comparing the mass-lost to the energy of the emitted gamma ray associated with the neutron capture. The binding mass-loss agreed with the gamma ray energy to a precision of ±0.00004 %, the most accurate test of E=mc<sup>2</sup> to date.<ref name="rainville"/>
| + | |
| | | | |
| − | The mass–energy equivalence formula was used in the understanding of [[nuclear fission]] reactions, and implies the great amount of energy that can be released by a [[nuclear fission]] [[chain reaction]], used in both [[nuclear weapon]]s and [[nuclear power]]. By measuring the mass of different [[atomic nuclei]] and subtracting from that number the total mass of the [[protons]] and [[neutrons]] as they would weigh separately, one gets the exact [[binding energy]] available in an [[atomic nucleus]]. This is used to calculate the energy released in any [[nuclear reaction]], as the difference in the total mass of the nuclei that enter and exit the reaction.
| + | :::::<math>{}_3^7\mathrm{Li}\, +\, {}_1^1\mathrm{H}\,\rightarrow\,2\, {}_2^4\mathrm{He}</math> |
| | | | |
| − | == Practical examples ==
| + | The mass of the particles on the left hand side is 8.0263 [[atomic mass units|amu]], the mass on the right hand side ''only'' 8.0077 amu.<ref>Gerard Piel ''The age of science: what scientists learned in the 20th century'', Basic Books, 2001, p. 144-145</ref> The difference between this masses is .00186 amu, which results in the following back-of-an-envelope calculation: |
| − | {{unreferenced section|date=August 2012}}
| + | |
| − | Einstein used the [[Centimeter gram second system of units|CGS]] system of units (centimeters, grams, seconds, dynes, and ergs), but the formula is independent of the system of units. In [[natural units]], the speed of light is defined to equal 1, and the formula expresses an identity: ''E'' = ''m''. In the [[International System of Units|SI]] system (expressing the ratio ''E'' / ''m'' in [[joules]] per kilogram using the value of ''c'' in [[metre per second|meters per second]]):{{citation needed|date=August 2012}}
| + | |
| | | | |
| − | :''E'' / ''m'' = ''c''<sup>2</sup> = (299,792,458 m/s or 983,571,056 ft/s)<sup>2</sup> = 89,875,517,873,681,764 J/kg (≈9.0 × 10<sup>16</sup> joules per kilogram). | + | ::::<math>0.00186\,\mathrm{amu} \cdot c^2 = 0.0186 \cdot 1.66 \cdot 10^{-27}\,\mathrm{kg}\cdot\left(3\cdot10^8\,\mathrm{\frac{m}{s}}\right)^2</math> |
| | + | ::::<math>\approx\,2.79\cdot 10^{-12} \,\mathrm{kg}\mathrm{\frac{m^2}{s^2}}</math> |
| | + | ::::<math>\approx \,17.3\,\mathrm{MeV}</math> |
| | | | |
| − | So the energy equivalent of one [[gram]] (1/1000 of a kilogram) of mass is equivalent to:
| + | Accurate measurements and detailed calculations allowed for verifying the theoretical values with an accuracy of ±0.5%. This was the first time a nucleus was artificially split, and thereby the first transmutation of elements using accelerated particles: |
| − | :89.9 [[joules|terajoules]]
| + | |
| − | :25.0 million [[kilowatt-hour]]s (≈25 [[GW·h]])
| + | |
| − | :21.5 billion [[calorie|kilocalories]] (≈21 Tcal)<sup> </sup><ref name="Conversion">Conversions used: 1956 International (Steam) Table (IT) values where one calorie ≡ 4.1868 J and one BTU ≡ 1055.05585262 J. Weapons designers' conversion value of one gram TNT ≡ 1000 calories used.<sup> </sup></ref>
| + | |
| − | :85.2 billion [[British thermal unit|BTUs]]<ref name="Conversion"/>
| + | |
| − | or to the energy released by combustion of the following:
| + | |
| − | :21.5 [[kiloton]]s of [[TNT equivalent|TNT-equivalent]] energy (≈21 kt)<sup><span style="font-size:100%;"> </span></sup><ref name="Conversion"/>
| + | |
| − | :568,000 US [[Imperial_gallon#Volume|gallons]] of automotive [[Gasoline#Energy_content_.28high_and_low_heating_value.29|gasoline]]
| + | |
| | | | |
| − | Any time energy is generated, the process can be evaluated from an ''E'' = ''mc''<sup>2</sup> perspective. For instance, the "[[Fat Man|Gadget]]"-style bomb used in the [[Trinity test]] and the [[bombing of Nagasaki]] had an explosive yield equivalent to 21 kt of TNT. About 1 kg of the approximately 6.15 kg of plutonium in each of these bombs fissioned into lighter elements totaling almost exactly one gram less, after cooling. The electromagnetic radiation and kinetic energy (thermal and blast energy) released in this explosion carried the missing one gram of mass.<ref>The 6.2 kg core comprised 0.8% gallium by weight. Also, about 20% of the Gadget's yield was due to fast fissioning in its natural uranium tamper. This resulted in 4.1 moles of Pu fissioning with 180 MeV per atom actually contributing prompt kinetic energy to the explosion. Note too that the term ''"Gadget"-style'' is used here instead of "Fat Man" because this general design of bomb was very rapidly upgraded to a more efficient one requiring only 5 kg of the Pu/gallium alloy.{{citation needed|date=August 2012}}</ref> This occurs because nuclear [[binding energy]] is released whenever elements with more than 62 nucleons fission.{{citation needed|date=August 2012}}
| + | ==A Famous Example -- Nuclear Fission of Uranium== |
| | | | |
| − | Another example is [[hydroelectricity|hydroelectric generation]]. The electrical energy produced by [[Grand Coulee Dam|Grand Coulee Dam's]] [[Water turbine|turbines]] every 3.7 hours represents one gram of mass. This mass passes to the electrical devices (such as lights in cities) which are powered by the generators, where it appears as a gram of heat and light.<ref>Assuming the dam is generating at its peak capacity of 6,809 MW.{{citation needed|date=August 2012}}</ref> Turbine designers look at their equations in terms of pressure, torque, and RPM. However, Einstein's equations show that all energy has mass, and thus the electrical energy produced by a dam's generators, and the heat and light which result from it, all retain their mass, which is equivalent to the energy. The potential energy—and equivalent mass—represented by the waters of the [[Columbia River]] as it descends to the Pacific Ocean would be converted to heat due to [[viscous friction]] and the [[turbulence]] of white water rapids and waterfalls were it not for the dam and its generators. This heat would remain as mass on site at the water, were it not for the equipment which converted some of this potential and kinetic energy into electrical energy, which can be moved from place to place (taking mass with it).{{citation needed|date=August 2012}}
| + | For most types of physical interactions, the masses of the initial reactants and of the final products match so closely that it is essentially impossible to measure any difference. But for nuclear reactions, the difference is measurable. That difference is related to the energy absorbed or released, described by the equation E=mc². (The equation applies to '''all''' interactions; the fact that nuclear interactions are the only ones for which the mass difference is measurable has led people to believe, wrongly, that E=mc² applies only to nuclear interactions.) |
| | | | |
| − | Whenever energy is added to a system, the system gains mass:{{citation needed|date=August 2012}}
| + | The [[Theory of Relativity]] played no role in this work, but proponents later tried to retrofit the theory to the data in order to explain the explain the observed mass changes.<ref>Actually, the formula E=mc<sup>2</sup> was published in 1905, and has not changed since then. Fission of Uranium was discovered in 1938. It is not possible that the equation was retrofitted to explain this discovery.</ref> Here is the most famous example of the mass change. |
| − | *A spring's mass increases whenever it is put into compression or tension. Its added mass arises from the added potential energy stored within it, which is bound in the stretched chemical (electron) bonds linking the atoms within the spring.
| + | |
| − | *Raising the temperature of an object (increasing its heat energy) increases its mass. For example, consider the world's primary mass standard for the kilogram, made of platinum/iridium. If its temperature is allowed to change by 1°C, its mass will change by 1.5 picograms (1 pg = 1 × 10<sup>−12</sup> g).<ref>Assuming a 90/10 alloy of Pt/Ir by weight, a ''C<sub>p</sub>'' of 25.9 for Pt and 25.1 for Ir, a Pt-dominated average ''C<sub>p</sub>'' of 25.8, 5.134 moles of metal, and 132 J.K<sup>-1</sup> for the prototype. A variation of ±1.5 picograms is of course, much smaller than the actual uncertainty in the mass of the international prototype, which is ±2 micrograms.</ref>
| + | |
| − | *A spinning ball will weigh more than a ball that is not spinning. Its increase of mass is exactly the equivalent of the mass of energy of rotation, which is itself the sum of the kinetic energies of all the moving parts of the ball. For example, [[the Earth]] itself is more massive due to its daily rotation, than it would be with no rotation. This rotational energy (2.14 x 10<sup>29</sup> J) represents 2.38 billion metric tons of added mass.<ref>InfraNet Lab (2008-12-07). Harnessing the Energy from the Earth's Rotation. Article on Earth rotation energy. Divided by c^2. InfraNet Lab, 7 December 2008. Retrieved from http://infranetlab.org/blog/2008/12/harnessing-the-energy-from-the-earth%E2%80%99s-rotation/.</ref>
| + | |
| | | | |
| − | Note that no net mass or energy is really created or lost in any of these examples and scenarios. Mass/energy simply moves from one place to another. These are some examples of the ''transfer'' of energy and mass in accordance with the ''principle of mass–energy conservation''.{{citation needed|date=August 2012}}
| + | Nuclear fission, which is the basis for nuclear energy, was discovered in experiments by [[Otto Hahn]] and [[Fritz Strassman]], and analyzed by [[Lise Meitner]], in 1938. |
| | | | |
| − | == Efficiency ==
| + | There are a great number of decay paths of [[Uranium]] fission that figured in this experiment. The result element that most caught their attention was [[Barium]], because it was chemically related to the Radium that they were expecting. One of the fission paths may have been this: |
| | | | |
| − | Although mass cannot be converted to energy, matter particles can be. Also, a certain amount of the ill-defined "matter" in ordinary objects can be converted to active energy (light and heat), even though no identifiable real particles are destroyed. Such conversions happen in nuclear weapons, in which the protons and neutrons in atomic nuclei lose a small fraction of their average mass, but this mass-loss is not due to the destruction of any protons or neutrons (or even, in general, lighter particles like electrons). Also the mass is not destroyed, but simply removed from the system in the form of heat and light from the reaction.
| + | :<sup>235</sup>U → <sup>140</sup>Xe + <sup>91</sup>Sr + 4n |
| | | | |
| − | In nuclear reactions, typically only a small fraction of the total mass–energy of the bomb is converted into heat, light, radiation and motion, which are "active" forms which can be used. When an atom fissions, it loses only about 0.1% of its mass (which escapes from the system and does not disappear), and in a bomb or reactor not all the atoms can fission. In a fission based atomic bomb, the [[Nuclear weapon design#Efficiency|efficiency]] is only 40%, so only 40% of the fissionable atoms actually fission, and only 0.04% of the total mass appears as energy in the end. In nuclear fusion, more of the mass is released as usable energy, roughly 0.3%. But in a fusion bomb (see [[nuclear weapon yield]]), the bomb mass is partly casing and non-reacting components, so that in practicality, no more than about 0.03% of the total mass of the entire weapon is released as usable energy (which, again, retains the "missing" mass).
| + | (The [[Xenon]] decayed within about a minute to <sup>140</sup>Ba. They were searching for the chemical signature of Barium.) |
| | | | |
| − | In theory, it should be possible to convert all of the mass in matter into heat and light (which would of course have the same mass), but none of the theoretically known methods are practical. One way to convert all matter into usable energy is to annihilate matter with [[antimatter]]. But [[baryon asymmetry|antimatter is rare in our universe]], and must be made first. Due to inefficient mechanisms of production, making antimatter always requires far more energy than would be released when it was annihilated.
| + | The masses of the particles are: |
| | | | |
| − | Since most of the mass of ordinary objects resides in protons and neutrons, in order to convert all ordinary matter to useful energy, the protons and neutrons must be converted to lighter particles. In the [[standard model|standard model of particle physics]], the [[baryon number|number of protons plus neutrons]] is nearly exactly conserved. Still, [[Gerard 't Hooft]] showed that there is a process which will convert protons and neutrons to antielectrons and neutrinos.<ref>G. 't Hooft, "Computation of the quantum effects due to a four-dimensional pseudoparticle", Physical Review D14:3432–3450 (1976).</ref> This is the weak SU(2) [[instanton]] proposed by Belavin Polyakov Schwarz and Tyupkin.<ref>A. Belavin, A. M. Polyakov, A. Schwarz, Yu. Tyupkin, "Pseudoparticle Solutions to Yang Mills Equations", Physics Letters 59B:85 (1975).</ref> This process, can in principle convert all the mass of matter into neutrinos and usable energy, but it is normally extraordinarily slow. Later it became clear that this process will happen at a fast rate at very high temperatures,<ref>F. Klinkhammer, [[N. Manton]], "A Saddle Point Solution in the Weinberg Salam Theory", Physical Review D 30:2212.</ref> since then instanton-like configurations will be copiously produced from [[statistical mechanics|thermal fluctuations]]. The temperature required is so high that it would only have been reached shortly after the [[big bang]].
| + | {| class="wikitable" border="1" cellpadding="8" cellspacing="0" |
| | + | ! Substance |
| | + | ! <sup>235</sup>U |
| | + | ! <sup>140</sup>Xe |
| | + | ! <sup>91</sup>Sr |
| | + | ! 4 neutrons |
| | + | |- |
| | + | | Number of protons |
| | + | | 92 |
| | + | | 54 |
| | + | | 38 |
| | + | | 0 |
| | + | |- |
| | + | | Number of neutrons |
| | + | | 235 |
| | + | | 140 |
| | + | | 91 |
| | + | | 4 |
| | + | |- |
| | + | | Number of electrons |
| | + | | 92 |
| | + | | 54 |
| | + | | 38 |
| | + | | 0 |
| | + | |- |
| | + | | Mass |
| | + | | 235.04393 |
| | + | | 139.92164 |
| | + | | 90.910203 |
| | + | | 4.03466 |
| | + | |} |
| | | | |
| − | Many extensions of the standard model contain [[magnetic monopole]]s, and in some models of [[grand unification theory|grand unification]], these monopoles catalyze [[proton decay]], a process known as the Callan–Rubakov effect.<ref>Rubakov V. A. "Monopole Catalysis of Proton Decay", Reports on Progress in Physics 51:189–241 (1988).</ref> This process would be an efficient mass–energy conversion at ordinary temperatures, but it requires making [[Magnetic monopole|monopole]]s and anti-monopoles first. The energy required to produce monopoles is believed to be enormous, but magnetic charge is conserved, so that the lightest monopole is stable. All these properties are deduced in theoretical models—magnetic monopoles have never been observed, nor have they been produced in any experiment so far.
| + | The mass of the Uranium atom is 235.04393, and the sum of the masses of the products is 234.866503. The difference is .177427 amu, or, using the E=mc² equation, 165 million electron volts. (The generally accepted value for the total energy released by Uranium fission, including secondary decays, is about 200 million electron volts.) |
| | | | |
| − | A third known method of total matter–energy conversion is using gravity, specifically black holes. [[Stephen Hawking]] theorized<ref>S.W. Hawking "Black Holes Explosions?" ''Nature'' 248:30 (1974).</ref> that black holes radiate thermally with no regard to how they are formed. So it is theoretically possible to throw matter into a black hole and use the emitted heat to generate power. According to the theory of [[Hawking radiation]], however, the black hole used will radiate at a higher rate the smaller it is, producing usable powers at only small black hole masses, where usable may for example be something greater than the local background radiation. It is also worth noting that the ambient irradiated power would change with the mass of the black hole, increasing as the mass of the black hole decreases, or decreasing as the mass increases, at a rate where power is proportional to the inverse square of the mass. In a "practical" scenario, mass and energy could be dumped into the black hole to regulate this growth, or keep its size, and thus power output, near constant. This could result from the fact that mass and energy are lost from the hole with its thermal radiation.
| + | The insight that the conversion from Uranium to Barium was caused by complete fission of the atom was made by Lise Meitner in December, 1938. She had the approximate "mass defect" quantities memorized, and so she worked out in her head, using the E=mc² equation, that there would be this enormous release of energy. This release was observed shortly thereafter, and the result is nuclear power and nuclear weapons. |
| | | | |
| − | ==Background== | + | ==A Topical Example: Speed of Extremely Energetic Neutrinos== |
| | + | Here is another example of the use of this formula in physics calculations. In 2011 there were [https://www.theguardian.com/science/2011/sep/22/faster-than-light-particles-neutrinos?newsfeed=true reports] that high-energy neutrinos had been observed traveling at a speed faster than the speed of light in an experiment at the Gran Sasso laboratory in Italy. Specifically, they seemed to have arrived at the detector 60 nanoseconds faster than light would have. Relativity doesn't allow that, and, since neutrinos have nonzero (but incredibly tiny) mass, they aren't even supposed to travel ''at'' the speed of light. |
| | | | |
| − | ===Mass–velocity relationship=== | + | The mass of a neutrino is about 0.44x10<sup>−36</sup>kilograms. (Normally all of these things are measured in more convenient units such as Giga-electron-Volts, but that makes implicit use of E=mc<sup>2</sup>. If we don't accept that, we have to do the calculations under classical physics, using SI (meter/kilogram/second) units.) The neutrinos were accelerated to an energy of about 17GeV, or .27x10<sup>−8</sup>Joules. If one did not accept relativity and had to use classical physics and the classical formula <math>\mathrm{E} = \frac{1}{2}mv^2</math>, one would get v=110x10<sup>12</sup> meters per second. This is about 370,000 times the speed of light, something that scientists would certainly have noticed. In fact, with special relativity, the speed is just under the speed of light, such that the neutrinos should be received at the detector about .26x10<sup>−24</sup> seconds (.26 yoctoseconds) later than the speed of light itself. This is far too small to measure—15 orders of magnitude smaller than the resolution of the GPS signals in the experiment. |
| | | | |
| − | In developing [[special relativity]], Einstein found that the [[kinetic energy#Relativistic kinetic energy of rigid bodies|kinetic energy]] of a moving body is
| + | Later [http://news.sciencemag.org/2012/02/official-word-superluminal-neutrinos-leaves-warp-drive-fans-shred-hope%E2%80%94barely?ref=hp reports] started to resolve the mystery, and it is now accepted that the neutrinos behaved properly. But a BBC reporter made the incorrect statement that [https://www.bbc.co.uk/news/science-environment-17364682 the neutrinos travelled at precisely light speed]. This was a simple misstatement, by .26 yoctoseconds. |
| | | | |
| − | ::<math>E_k = m_0 ( \gamma -1 ) c^2 = \frac{m_0 c^2}{\sqrt{1-\frac{v^2}{c^2}}} - m_0 c^2,</math> | + | The issue was discussed at length at Conservapedia.<ref>http://www.conservapedia.com/Talk:Main_Page/Archive_index/102#Faster_than_light_neutrinos</ref><ref>http://www.conservapedia.com/Talk:Main_Page/Archive_index/102#The_final_nail_in_the_coffin_of_relativity.3F</ref><ref>http://www.conservapedia.com/Talk:Main_Page/Archive_index/102#Another_Blow_to_Relativity</ref><ref>http://www.conservapedia.com/Talk:Main_Page/Archive_index/109#Neutrinos_now_obey_speed_limit</ref><ref>http://www.conservapedia.com/Talk:Main_Page/Archive_index/111#Neutrinos</ref> |
| | | | |
| − | with <math>v</math> the [[velocity]], <math>m_0</math> the rest mass, and γ the [[Lorentz factor]].
| + | ==Deducing the Equation From Empirical Observation== |
| | | | |
| − | He included the second term on the right to make sure that for small velocities, the energy would be the same as in classical mechanics:
| + | While the equation was historically developed on theoretical grounds as an inevitable consequence of special relativity, it is possible to deduce it purely from empirical observation. |
| | | | |
| − | ::<math>E_k = \frac{1}{2}m_0 v^2 + \cdots </math>
| + | So, for the purposes of this section, imagine that one is in the era of "classical physics"; prior to 1900 or so. Relativity has not been invented, but, inexplicably, nuclear physics has. Imagine that the phenomena of radioactivity and nuclear fission have been observed, without any knowledge of relativity. |
| | | | |
| − | Without this second term, there would be an additional contribution in the energy when the particle is not moving.
| + | A well-accepted physical law of classical physics was the law of conservation of mass. This was not easy to deduce. It required careful analysis of such phenomena as combustion, in the 1700s, to eliminate the various confounding sub-phenomena that made the law difficult to see. But, by 1900, the law was well established: |
| | | | |
| − | Einstein found that the [[Momentum#Modern definitions of momentum|total momentum]] of a moving particle is:
| + | :::*'''In all interactions, mass is precisely conserved.''' |
| | | | |
| − | ::<math>P = \frac{m_0 v}{\sqrt{1-\frac{v^2}{c^2}}}. </math>
| + | For example, the mass of a TNT molecule is 227.1311 Daltons, or 227.1311 g/mol, which is, for all practical purposes, the same as the mass of its constituent Carbon, Hydrogen, Nitrogen, and Oxygen atoms. It is essentially impossible to measure the difference. The principle of conservation of mass is upheld. |
| | | | |
| − | and it is this quantity which is conserved in collisions. The ratio of the momentum to the velocity is the [[relativistic mass]], m.
| + | But when nuclear phenomena are discovered, we notice something different. The masses of the result particles after an event (e.g. alpha decay, nuclear fission, or artificial transmutation) is measurably less than the masses of the original particle(s). With the invention of the mass spectrometer around 1920, it became possible to measure atomic weights of various isotopes with great precision. |
| | | | |
| − | ::<math>m = \frac{m_0}{\sqrt{1-\frac{v^2}{c^2}}}</math>
| + | Radium-226 decays into Radon-222 by emission of an alpha particle with an energy of 4.78 MeV. |
| | | | |
| − | And the relativistic mass and the relativistic kinetic energy are related by the formula:
| + | 1 kg of Radium-226 = <math>\frac{6.022 \times 10^{23}}{226.0254}</math> atoms. (The numerator is [[Avogadro's number]], and the denominator is the atomic weight of Radium-226.) This is 2.6643647 * 10<sup>24</sup> atoms. |
| | | | |
| − | ::<math>E_k = m c^2 - m_0 c^2. \,</math>
| + | That number of Radon-222 atoms has mass .98226836 kg. That number of alpha particles has mass .01770863 kg. |
| | + | The mass lost is .00002301 kg. |
| | | | |
| − | Einstein wanted to omit the unnatural second term on the right-hand side, whose only purpose is to make the energy at rest zero, and to declare that the particle has a total energy which obeys:
| + | Each emitted alpha particle has energy of 4.78 MeV, or 4.78 * .1602 * 10<sup>−18</sup> Joules. The total alpha energy from the decay of 1 kg of radium is 2.04 * 10<sup>12</sup> Joules. |
| − | ::<math> E = m c^2 \,</math>
| + | |
| | | | |
| − | which is a sum of the rest energy ''m''<sub>0</sub>''c''<sup>2</sup> and the kinetic energy. This total energy is mathematically more elegant, and fits better with the momentum in relativity. But to come to this conclusion, Einstein needed to think carefully about collisions. This expression for the energy implied that matter at rest has a huge amount of energy, and it is not clear whether this energy is physically real, or just a mathematical artifact with no physical meaning.
| |
| | | | |
| − | In a collision process where all the rest-masses are the same at the beginning as at the end, either expression for the energy is conserved. The two expressions only differ by a constant which is the same at the beginning and at the end of the collision. Still, by analyzing the situation where particles are thrown off a heavy central particle, it is easy to see that the inertia of the central particle is reduced by the total energy emitted. This allowed Einstein to conclude that the inertia of a heavy particle is increased or diminished according to the energy it absorbs or emits.
| + | Also, Radon-222 decays into Polonium-218 by emission of an alpha particle with an energy of 5.49 MeV. |
| | | | |
| − | ===Relativistic mass=== | + | 1 kg of Radon-222 = <math>\frac{6.022 \times 10^{23}}{222.0176}</math> atoms. This is 2.7124611 * 10<sup>24</sup> atoms. |
| − | {{Main|Mass in special relativity}} | + | |
| | | | |
| − | After Einstein first made his proposal, it became clear that the word mass can have two different meanings. The rest mass is what Einstein called ''m'', but others defined the ''relativistic mass'' with an explicit index:
| + | That number of Polonium-218 atoms has mass .98194467 kg. That number of alpha particles has mass .01802830 kg. |
| | | | |
| − | ::<math>m_{\mathrm{rel}} = \frac{m_0}{\sqrt{1-\frac{v^2}{c^2}}}\,\, . </math>
| + | The mass lost is .00002703 kg. |
| | | | |
| − | This mass is the ratio of momentum to velocity, and it is also the relativistic energy divided by {{math|1=''c''<sup>2</sup>}} (it is not Lorentz-invariant, in contrast to <math>m_0</math>). The equation {{math|1=''E'' = ''m''<sub>rel</sub>''c''<sup>2</sup>}} holds for moving objects. When the velocity is small, the relativistic mass and the rest mass are almost exactly the same.
| + | Each emitted alpha particle has energy of 5.49 MeV. The total alpha energy from the decay of 1 kg of polonium is 2.39 * 10<sup>12</sup> Joules. |
| | | | |
| − | *''E''=''mc''<sup>2</sup> either means ''E''=''m''<sub>0</sub>''c''<sup>2</sup> for an object at rest, or ''E''=''m''<sub>rel</sub>''c''<sup>2</sup> when the object is moving.
| + | It looks as thought we have to rewrite the law of conservation of mass: |
| | | | |
| − | Also Einstein (following [[Hendrik Lorentz]] and [[Max Abraham]]) used velocity—and direction-dependent mass concepts ([[Mass in special relativity#Early developments: transverse and longitudinal mass|longitudinal and transverse mass]]) in his 1905 electrodynamics paper and in another paper in 1906.<ref>{{Citation | author=Einstein, A. | year=1905 | title=Zur Elektrodynamik bewegter Körper. | journal=Annalen der Physik | volume=17 | pages=891–921 | doi=10.1002/andp.19053221004 |url=http://www.physik.uni-augsburg.de/annalen/history/papers/1905_17_891-921.pdf|format=PDF|bibcode = 1905AnP...322..891E }}. [http://www.fourmilab.ch/etexts/einstein/specrel/www/ English translation.]</ref><ref>{{Citation | author=Einstein, A. | year=1906 | title=Über eine Methode zur Bestimmung des Verhältnisses der transversalen und longitudinalen Masse des Elektrons. | journal=Annalen der Physik | volume=21 | pages=583–586 | doi=10.1002/andp.19063261310| url=http://www.physik.uni-augsburg.de/annalen/history/papers/1906_21_583-586.pdf|format=PDF|bibcode = 1906AnP...326..583E }}</ref>
| + | :::*'''In all "ordinary" interactions, mass is precisely conserved.''' |
| − | However, in his first paper on ''E''=''mc''<sup>2</sup> (1905), he treated ''m'' as what would now be called the ''rest mass''.<ref name="inertia" /> Some claim that (in later years) he did not like the idea of "relativistic mass."<ref>See e.g. Lev B.Okun, ''The concept of Mass'', Physics Today '''42''' (6), June 1969, p. 31–36, http://www.physicstoday.org/vol-42/iss-6/vol42no6p31_36.pdf</ref> When modern physicists say "mass", they are usually talking about rest mass, since if they meant "relativistic mass", they would just say "energy".
| + | :::*'''In nuclear interactions, there is a small but measurable loss of mass.''' |
| | | | |
| − | Considerable debate has ensued over the use of the concept "relativistic mass" and the connection of "mass" in relativity to "mass" in Newtonian dynamics. For example, one view is that only rest mass is a viable concept and is a property of the particle; while relativistic mass is a conglomeration of particle properties and properties of spacetime. A perspective that avoids this debate, due to Kjell Vøyenli, is that the Newtonian concept of mass as a particle property and the relativistic concept of mass have to be viewed as embedded in their own theories and as having no precise connection.<ref name="Jammer">
| + | :By the way, we can clearly see that atomic weights of pure isotopes are not integers, and that it has something to do with the energy released by nuclear disintegration. In retrospect, the formula E=mc² explains the non-integer character of atomic weights. |
| | | | |
| − | {{Citation |title=Concepts of mass in contemporary physics and philosophy |author=Max Jammer |url=http://books.google.com/?id=jujK1bn4QUQC&pg=PA51 |page=51 |isbn=0-691-01017-X |publisher=Princeton University Press |year=1999 }}</ref><ref name="Vøyenli">
| + | Making special cases like this is unsatisfactory, of course. |
| | | | |
| − | {{Citation |journal=Foundations of Physics |doi=10.1007/BF00708670 |title= The classical and relativistic concepts of mass |year=1976 |author= Eriksen, Erik; Vøyenli, Kjell |volume=6 |pages=115–124 |publisher=Springer |bibcode=1976FoPh....6..115E}}</ref>
| + | We do this for a few other interactions, including the explosion of TNT. This would include the Lithium-plus-Hydrogen and Uranium fission phenomena described above. We won't bother with the details. As observational scientists, we look for patterns in the behavior of nature. We make a table: |
| | | | |
| − | === Low-speed expansion === | + | {| class="wikitable" border="1" cellpadding="8" cellspacing="0" |
| | + | ! interaction |
| | + | ! energy released per kg, Joules |
| | + | ! mass lost per kg of original substance, kg |
| | + | |- |
| | + | | explosion of TNT |
| | + | | 4.184 * 10<sup>6</sup> |
| | + | | seems to be zero |
| | + | |- |
| | + | | alpha decay of Ra-226 |
| | + | | 2.04 * 10<sup>12</sup> |
| | + | | .00002301 kg |
| | + | |- |
| | + | | alpha decay of Rn-222 |
| | + | | 2.39 * 10<sup>12</sup> |
| | + | | .00002703 kg |
| | + | |} |
| | | | |
| − | We can rewrite the expression ''E'' = ''γm''<sub>0</sub>''c''<sup>2</sup> as a [[Taylor series]]:
| |
| | | | |
| − | :<math>E = m_0 c^2 \left[1 + \frac{1}{2} \left(\frac{v}{c}\right)^2 + \frac{3}{8} \left(\frac{v}{c}\right)^4 + \frac{5}{16} \left(\frac{v}{c}\right)^6 + \ldots \right]. </math>
| + | We plot these, and a few others, not shown, on graph paper, and find to our amazement that the relationship is linear. |
| | | | |
| − | For speeds much smaller than the speed of light, higher-order terms in this expression get smaller and smaller because ''v''/''c'' is small. For low speeds we can ignore all but the first two terms: | + | For Ra, m/E = .112794118 E-16 |
| | + | For Po, m/E = .113096234 E-16 |
| | | | |
| − | :<math>E \approx m_0 c^2 + \frac{1}{2} m_0 v^2 . </math>
| + | If this is linear, the mass defect for TNT would have been .47 * 10<sup>−10</sup>. We couldn't possibly have measured this. |
| | | | |
| − | The total energy is a sum of the rest energy and the [[classical mechanics|Newtonian]] [[kinetic energy]].
| + | So we can rewrite the rule for conservation of mass in a more satisfactory way: |
| | | | |
| − | The classical energy equation ignores both the ''m''<sub>0</sub>''c''<sup>2</sup> part, and the high-speed corrections. This is appropriate, because all the high-order corrections are small. Since only ''changes'' in energy affect the behavior of objects, whether we include the ''m''<sub>0</sub>''c''<sup>2</sup> part makes no difference, since it is constant. For the same reason, it is possible to subtract the rest energy from the total energy in relativity. By considering the emission of energy in different frames, Einstein could show that the rest energy has a real physical meaning.
| + | :::*'''In all interactions, there is a loss of mass, equal to about .113 * 10<sup>-16</sup> kg per Joule of energy released.''' |
| | | | |
| − | The higher-order terms are extra correction to Newtonian mechanics which become important at higher speeds. The Newtonian equation is only a low-speed approximation, but an extraordinarily good one. All of the calculations used in putting astronauts on the moon, for example, could have been done using Newton's equations without any of the higher-order corrections.
| + | What we thought was exact conservation is just very nearly exact, and we hadn't been able to measure it before. |
| | | | |
| − | ==History==
| + | But maybe there's more. This constant has dimensions of kilograms per Joule. From high-school physics, we know that that is seconds squared divided by meters squared. That is, it is the reciprocal of the square of a velocity. We calculate that velocity. It is about 2.97 * 10<sup>8</sup> meters per second. Very close to the speed of light! Very interesting! (The calculations above were not extremely precise. The formula has been verified with great precision, but not here.) |
| | | | |
| − | While Einstein was the first to have correctly deduced the mass–energy equivalence formula, he was not the first to have related energy with mass. But nearly all previous authors thought that the energy which contributes to mass comes only from electromagnetic fields.<ref name="jann">{{Citation | author=Jannsen, M., Mecklenburg, M. | year=2007 | contribution=From classical to relativistic mechanics: Electromagnetic models of the electron. | editor=V. F. Hendricks, et al.| journal=Interactions: Mathematics, Physics and Philosophy | pages=65–134 | place=Dordrecht | publisher=Springer | url =http://www.tc.umn.edu/~janss011/ }}</ref><ref name="whit">{{Citation | author=Whittaker, E.T. | year=1951–1953 | title= 2. Edition: A History of the theories of aether and electricity, vol. 1: The classical theories / vol. 2: The modern theories 1900–1926 | place=London | publisher=Nelson}}</ref><ref name="mill">{{Citation|author=Miller, Arthur I.|year=1981|title= Albert Einstein's special theory of relativity. Emergence (1905) and early interpretation (1905–1911)|place= Reading|publisher=Addison–Wesley|isbn=0-201-04679-2}}</ref><ref name="darr">{{Citation | author=Darrigol, O. | title=The Genesis of the theory of relativity. | year=2005 | journal=Séminaire Poincaré | volume=1 | pages=1–22| url=http://www.bourbaphy.fr/darrigol2.pdf|format=PDF}}</ref>
| + | We don't understand why (that will have to wait for the invention of relativity), but we can formulate a hypothesis: |
| | | | |
| − | ===Newton: matter and light===
| + | :::*'''In all interactions, there is a loss of mass, equal to <math>\frac{1}{c^2}</math> times the amount of energy released.''' |
| | | | |
| − | In 1717 [[Isaac Newton]] speculated that light particles and matter particles were inter-convertible in "Query 30" of the ''[[Opticks]]'', where he asks:
| + | We don't have to give the units any more, since everything is now dimensionally correct. |
| | | | |
| − | {{quote|Are not the gross bodies and light convertible into one another, and may not bodies receive much of their activity from the particles of light which enter their composition?}}
| + | ::There is a very interesting analogy with the discovery of [[Maxwell's Equations]]. Maxwell found an interesting relationship involving the fundamental constants <math>\epsilon\,</math> and <math>\mu\,</math> appearing in his equations. Specifically, <math>\epsilon\mu\,</math> has the dimensions of seconds squared divided by meters squared, and that: |
| | | | |
| − | ===Swedenborg: matter composed of "pure and total motion"===
| + | :::::<math>\frac{1}{\epsilon\mu} = c^2</math> |
| | | | |
| − | In 1734 [[Emanuel Swedenborg]] in his Principia theorized that all matter is ultimately composed of dimensionless points of "pure and total motion." He described this motion as being without force, direction or speed, but having the potential for force, direction and speed everywhere within it.<ref>{{cite book|last=Swedenborg|first=Emanuel|title=Principia Rerum Naturalia|year=1734|location=Leipzig|language=Latin|chapter=De Simplici Mundi vel Puncto naturali|page=32}}</ref><ref>{{cite book|last=Swedenborg|first=Emanuel|title=The Principia; or The First Principles of Natural Things|year=1845|publisher=W. Newbery|location=London|translator=Augustus Clissold|pages=55–57}}</ref>
| + | ::where "c" was the known velocity of light. He also showed that his equations predict electromagnetic waves, propagating at that speed. |
| − | | + | |
| − | ===Electromagnetic mass===
| + | |
| − | {{Main|Electromagnetic mass}}
| + | |
| − | There were many attempts in the 19th and the beginning of the 20th century—like those of [[J. J. Thomson]] (1881), [[Oliver Heaviside]] (1888), and [[George Frederick Charles Searle]] (1897), [[Wilhelm Wien]] (1900), [[Max Abraham]] (1902), [[Hendrik Antoon Lorentz]] (1904) — to understand as to how the mass of a charged object depends on the electrostatic field.<ref name="jann"/><ref name="whit"/>
| + | |
| − | This concept was called [[electromagnetic mass]], and was considered as being dependent on velocity and direction as well. Lorentz (1904) gave the following expressions for longitudinal and transverse electromagnetic mass:
| + | |
| − | :<math>m_{L}=\frac{m_{0}}{\left(\sqrt{1-\frac{v^{2}}{c^{2}}}\right)^{3}},\quad m_{T}=\frac{m_{0}}{\sqrt{1-\frac{v^{2}}{c^{2}}}} </math>, | + | |
| − | | + | |
| − | where | + | |
| − | :<math>m_{0}=\frac{4}{3}\frac{E_{em}}{c^{2}}</math>
| + | |
| − | | + | |
| − | ===Radiation pressure and inertia===
| + | |
| − | {{Main|Electromagnetic mass#Inertia of energy and radiation paradoxes}}
| + | |
| − | Another way of deriving some sort of electromagnetic mass was based on the concept of [[radiation pressure]]. In 1900, [[Henri Poincaré]] associated electromagnetic radiation energy with a "fictitious fluid" having momentum and mass <math>m_{em}=E_{em}/c^2</math>. By that, Poincaré tried to save the [[center of mass]] theorem in Lorentz's theory, though his treatment led to radiation paradoxes.<ref name="darr" />
| + | |
| − | | + | |
| − | [[Friedrich Hasenöhrl]] showed in 1904, that electromagnetic [[cavity radiation]] contributes the "apparent mass" <math>m_{0}=\tfrac{4}{3}\tfrac{E_{em}}{c^{2}}</math> to the cavity's mass. He argued that this implies mass dependence on temperature as well.<ref>{{Cite web|author=Philip Ball|title=Did Einstein discover E = mc2?|publisher=[[Physics World]]|date=Aug 23, 2011|url=http://physicsworld.com/cws/article/news/46941}}</ref>
| + | |
| − | | + | |
| − | ===Einstein: mass–energy equivalence===
| + | |
| − | | + | |
| − | [[Albert Einstein]] did not formulate exactly the formula {{nowrap|1=''E'' = ''mc''<sup>2</sup>}} in his 1905 [[Annus Mirabilis Papers|''Annus Mirabilis'' paper]] "Does the Inertia of a Body Depend Upon Its Energy Content?";<ref name="inertia" /> rather, the paper states that if a body gives off the energy ''L'' in the form of radiation, its mass diminishes by ''L''/''c''<sup>2</sup>. (Here, "radiation" means [[electromagnetic radiation]], or light, and mass means the ordinary Newtonian mass of a slow-moving object.) This formulation relates only a change Δ''m'' in mass to a change ''L'' in energy without requiring the absolute relationship.
| + | |
| − |
| + | |
| − | Objects with zero mass presumably have zero energy, so the extension that all mass is proportional to energy is obvious from this result. In 1905, even the hypothesis that changes in energy are accompanied by changes in mass was untested. Not until the discovery of the first type of antimatter (the [[positron]] in 1932) was it found that all of the mass of pairs of resting particles could be converted to radiation.
| + | |
| − | | + | |
| − | ====First derivation (1905)====
| + | |
| − | | + | |
| − | Already in his relativity paper "On the electrodynamics of moving bodies", Einstein derived the correct expression for the kinetic energy of particles:
| + | |
| − | | + | |
| − | :<math>E_{k}=mc^{2}\left(\frac{1}{\sqrt{1-\frac{v^{2}}{c^{2}}}}-1\right)</math>.
| + | |
| − | | + | |
| − | Now the question remained open as to which formulation applies to bodies at rest. This was tackled by Einstein in his paper "Does the inertia of a body depend upon its energy content?". Einstein used a body emitting two light pulses in opposite directions, having energies of <math>E_0</math> before and <math>E_1</math> after the emission as seen in its rest frame. As seen from a moving frame, this becomes <math>H_0</math> and <math>H_1</math>. Einstein obtained:
| + | |
| − | | + | |
| − | :<math>\left(H_{0}-E_{0}\right)-\left(H_{1}-E_{1}\right)=E\left(\frac{1}{\sqrt{1-\frac{v^{2}}{c^{2}}}}-1\right)</math>
| + | |
| − | | + | |
| − | then he argued that <math>H-E</math> can only differ from the kinetic energy <math>K</math> by an additive constant, which gives
| + | |
| − | | + | |
| − | :<math>K_{0}-K_{1}=E\left(\frac{1}{\sqrt{1-\frac{v^{2}}{c^{2}}}}-1\right)</math>
| + | |
| − | | + | |
| − | Neglecting effects higher than third order in <math>v/c</math> this gives:
| + | |
| − | | + | |
| − | :<math>K_{0}-K_{1}=\frac{E}{c^{2}}\frac{v^{2}}{2}</math>
| + | |
| − | | + | |
| − | Thus Einstein concluded that the emission reduces the body's mass by <math>E/c^2</math>, and that the mass of a body is a measure of its energy content.
| + | |
| − | | + | |
| − | The correctness of Einstein's 1905 derivation of ''E''=''mc''<sup>2</sup> was criticized by [[Max Planck]] (1907), who argued that it is only valid to first approximation. Another criticism was formulated by [[Herbert Ives]] (1952) and [[Max Jammer]] (1961), asserting that Einstein's derivation is based on [[begging the question]].<ref>{{Citation | author=Ives, Herbert E. | year=1952 | title=Derivation of the mass-energy relation | journal=Journal of the Optical Society of America | volume= 42 | issue=8 | pages =540–543 | doi=10.1364/JOSA.42.000540}}</ref><ref>{{Cite book|author=Jammer, Max|title=Concepts of Mass in Classical and Modern Physics|location=New York|publisher=Dover|year=1961/1997|isbn=0-486-29998-8}}</ref>
| + | |
| − | On the other hand, [[John Stachel]] and [[Roberto Torretti]] (1982) argued that Ives' criticism was wrong, and that Einstein's derivation was correct.<ref>{{Citation | author=Stachel, John; Torretti, Roberto | year=1982 | title=Einstein's first derivation of mass-energy equivalence | journal=American Journal of Physics | volume =50 | issue=8 | pages =760–763 | doi=10.1119/1.12764|bibcode = 1982AmJPh..50..760S }}</ref>
| + | |
| − | Hans Ohanian (2008) agreed with Stachel/Torretti's criticism of Ives, though he argued that Einstein's derivation was wrong for other reasons.<ref>{{Citation | author=Ohanian, Hans | year=2008 | title=Did Einstein prove E=mc2? | journal=Studies In History and Philosophy of Science Part B | volume =40 | issue=2 | pages =167–173 | doi=10.1016/j.shpsb.2009.03.002|arxiv=0805.1400}}</ref> For a recent review, see Hecht (2011).<ref>{{Citation | author=Hecht, Eugene | year=2011 | title=How Einstein confirmed E0=mc2 | journal=American Journal of Physics | volume =79 | issue=6 | pages =591–600 | doi=10.1119/1.3549223|bibcode = 2011AmJPh..79..591H }}</ref>
| + | |
| − | | + | |
| − | ====Alternative version====
| + | |
| − | An alternative version of Einstein's [[thought experiment]] was proposed by Fritz Rohrlich (1990), who based his reasoning on the [[Doppler effect]].<ref>{{Citation | author=Rohrlich, Fritz | year=1990 | title=An elementary derivation of E=mc2 | journal=American Journal of Physics | volume= 58 | issue=4 | pages =348–349 | doi=10.1119/1.16168|bibcode = 1990AmJPh..58..348R }}</ref>
| + | |
| − | Like Einstein, he considered a body at rest with mass ''M''. If the body is examined in a frame moving with nonrelativistic velocity ''v'', it is no longer at rest and in the moving frame it has momentum ''P'' = ''Mv''. Then he supposed the body emits two pulses of light to the left and to the right, each carrying an equal amount of energy ''E''/2. In its rest frame, the object remains at rest after the emission since the two beams are equal in strength and carry opposite momentum.
| + | |
| − | | + | |
| − | But if the same process is considered in a frame moving with velocity ''v'' to the left, the pulse moving to the left will be [[redshift]]ed while the pulse moving to the right will be [[blue shift]]ed. The blue light carries more momentum than the red light, so that the momentum of the light in the moving frame is not balanced: the light is carrying some net momentum to the right.
| + | |
| − | | + | |
| − | The object has not changed its velocity before or after the emission. Yet in this frame it has lost some right-momentum to the light. The only way it could have lost momentum is by losing mass. This also solves Poincaré's radiation paradox, discussed above.
| + | |
| − | | + | |
| − | The velocity is small, so the right-moving light is blueshifted by an amount equal to the nonrelativistic [[Doppler shift]] factor {{nowrap|1 − ''v''/''c''}}. The momentum of the light is its energy divided by ''c'', and it is increased by a factor of ''v''/''c''. So the right-moving light is carrying an extra momentum <math>\Delta P</math> given by:
| + | |
| − | | + | |
| − | :<math>
| + | |
| − | \Delta P = {v \over c}{E \over 2c}.
| + | |
| − | \,</math>
| + | |
| − | | + | |
| − | The left-moving light carries a little less momentum, by the same amount <math>\Delta P</math>. So the total right-momentum in the light is twice <math> \Delta P</math>. This is the right-momentum that the object lost.
| + | |
| − | | + | |
| − | :<math>
| + | |
| − | 2\Delta P = v {E\over c^2}.
| + | |
| − | \,</math>
| + | |
| − | | + | |
| − | The momentum of the object in the moving frame after the emission is reduced by this amount:
| + | |
| − | | + | |
| − | :<math>
| + | |
| − | P' = Mv - 2\Delta P = \left(M - {E\over c^2}\right)v.
| + | |
| − | \,</math>
| + | |
| − | | + | |
| − | So the change in the object's mass is equal to the total energy lost divided by <math>c^2</math>. Since any emission of energy can be carried out by a two step process, where first the energy is emitted as light and then the light is converted to some other form of energy, any emission of energy is accompanied by a loss of mass. Similarly, by considering absorption, a gain in energy is accompanied by a gain in mass.
| + | |
| − | | + | |
| − | ====Relativistic center-of-mass theorem – 1906====
| + | |
| − | | + | |
| − | Like Poincaré, Einstein concluded in 1906 that the inertia of electromagnetic energy is a necessary condition for the center-of-mass theorem to hold. On this occasion, Einstein referred to Poincaré's 1900 paper and wrote:<ref>{{Citation | author=Einstein, A. | year=1906 | title=Das Prinzip von der Erhaltung der Schwerpunktsbewegung und die Trägheit der Energie | journal=Annalen der Physik | volume =20 | pages =627–633 | doi=10.1002/andp.19063250814| url=http://www.physik.uni-augsburg.de/annalen/history/papers/1906_20_627-633.pdf|format=PDF|bibcode = 1906AnP...325..627E }}</ref>
| + | |
| − | | + | |
| − | {{quote|Although the merely formal considerations, which we will need for the proof, are already mostly contained in a work by H. Poincaré<sup>2</sup>, for the sake of clarity I will not rely on that work.<ref>Einstein 1906: Trotzdem die einfachen formalen Betrachtungen, die zum Nachweis dieser Behauptung durchgeführt werden müssen, in der Hauptsache bereits in einer Arbeit von H. Poincaré enthalten sind<sup>2</sup>, werde ich mich doch der Übersichtlichkeit halber nicht auf jene Arbeit stützen.</ref>}}
| + | |
| − | | + | |
| − | In Einstein's more physical, as opposed to formal or mathematical, point of view, there was no need for fictitious masses. He could avoid the ''[[perpetual motion|perpetuum mobile]]'' problem, because on the basis of the mass–energy equivalence he could show that the transport of inertia which accompanies the emission and absorption of radiation solves the problem. Poincaré's rejection of the principle of action–reaction can be avoided through Einstein's <math>E=mc^2</math>, because mass conservation appears as a special case of the [[energy conservation law]].
| + | |
| − | | + | |
| − | ===Others===
| + | |
| − | | + | |
| − | During the nineteenth century there were several speculative attempts to show that mass and energy were proportional in various [[Aether theories|ether theories]].<ref>Helge Kragh, "Fin-de-Siècle Physics: A World Picture in Flux" in ''Quantum Generations: A History of Physics in the Twentieth Century'' (Princeton, NJ: Princeton University Press, 1999.</ref> In 1873 [[Nikolay Umov]] pointed out a relation between mass and energy for ether in the form of ''Е = kmc''<sup>2</sup>, where 0.5 ≤ k ≤ 1.<ref>''Умов Н. А.'' Избранные сочинения. М. — Л., 1950. (Russian)</ref> The writings of [[Samuel Tolver Preston]],<ref>Preston, S. T., Physics of the Ether, E. & F. N. Spon, London, (1875).</ref><ref>Bjerknes: [http://itis.volta.alessandria.it/episteme/ep6/ep6-bjerk1.htm S. Tolver Preston's Explosive Idea ''E'' = ''mc''<sup>2</sup>.]</ref> and a 1903 paper by [[Olinto De Pretto]],<ref name="math">MathPages: [http://www.mathpages.com/rr/s8-08/8-08.htm Who Invented Relativity?]</ref><ref>De Pretto, O. ''Reale Instituto Veneto Di Scienze, Lettere Ed Arti'', LXIII, II, 439–500, reprinted in Bartocci.</ref> presented a mass–energy relation. De Pretto's paper received recent press coverage when [[Umberto Bartocci]] discovered that there were only [[six degrees of separation|three degrees of separation]] linking De Pretto to Einstein, leading Bartocci to conclude that Einstein was probably aware of De Pretto's work.<ref>Umberto Bartocci, ''Albert Einstein e Olinto De Pretto—La vera storia della formula più famosa del mondo'', editore Andromeda, Bologna, 1999.</ref>
| + | |
| − | | + | |
| − | Preston and De Pretto, following [[Le Sage's theory of gravitation|Le Sage]], imagined that the universe was filled with an ether of tiny particles which are always moving at speed ''c''. Each of these particles have a kinetic energy of ''mc''<sup>2</sup> up to a small numerical factor. The nonrelativistic kinetic energy formula did not always include the traditional factor of 1/2, since [[Gottfried Leibniz|Leibniz]] introduced kinetic energy without it, and the 1/2 is largely conventional in prerelativistic physics.<ref>{{Citation| author=Prentiss, J.J.| title=Why is the energy of motion proportional to the square of the velocity? | journal=American Journal of Physics | volume=73| issue = 8 | month=August | year=2005 | page=705}}</ref> By assuming that every particle has a mass which is the sum of the masses of the ether particles, the authors would conclude that all matter contains an amount of kinetic energy either given by ''E'' = ''mc''<sup>2</sup> or 2''E'' = ''mc''<sup>2</sup> depending on the convention. A particle ether was usually considered unacceptably speculative science at the time,<ref>John Worrall, review of the book ''Conceptions of Ether. Studies in the History of Ether Theories'' by Cantor and Hodges, The British Journal of the Philosophy of Science vol 36, no 1, March 1985, p. 84. The article contrasts a particle ether with a wave-carrying ether, the latter ''was'' acceptable.</ref> and since these authors did not formulate relativity, their reasoning is completely different from that of Einstein, who used relativity to change frames.
| + | |
| − | | + | |
| − | Independently, [[Gustave Le Bon]] in 1905 speculated that atoms could release large amounts of latent energy, reasoning from an all-encompassing qualitative philosophy of physics.<ref>Le Bon: [http://www.rexresearch.com/lebonfor/evforp1.htm#p1b3ch2 The Evolution of Forces.]</ref><ref>Bizouard: [http://www.annales.org/archives/x/poincaBizouard.pdf Poincaré ''E'' = ''mc''<sup>2</sup> l'équation de Poincaré, Einstein et Planck.]</ref>
| + | |
| − | | + | |
| − | ===Radioactivity and nuclear energy===
| + | |
| − | | + | |
| − | It was quickly noted after the discovery of [[radioactivity]] in 1897, that the total energy due to radioactive processes is about one ''million times'' greater than that involved in any known molecular change. However, it raised the question where this energy is coming from. After eliminating the idea of absorption and emission of some sort of [[Le Sage's theory of gravitation|Lesagian ether particles]], the existence of a huge amount of latent energy, stored within matter, was proposed by [[Ernest Rutherford]] and [[Frederick Soddy]] in 1903. Rutherford also suggested that this internal energy is stored within normal matter as well. He went on to speculate in 1904:<ref>{{Citation|last=Rutherford|first= Ernest |year=1904|title=Radioactivity |publisher=University Press|location=Cambridge|pages=336–338|url=http://www.archive.org/details/radioactivity00ruthrich}}</ref><ref>{{Citation|last=Heisenberg|first= Werner |year=1958|title=Physics And Philosophy: The Revolution In Modern Science |publisher=Harper & Brothers|location=New York|pages=118–119|url=http://www.archive.org/details/physicsandphilos010613mbp}}</ref>
| + | |
| − | | + | |
| − | {{quote|If it were ever found possible to control at will the rate of disintegration of the radio-elements, an enormous amount of energy could be obtained from a small quantity of matter.}}
| + | |
| − | | + | |
| − | Einstein's equation is in no way an explanation of the large energies released in radioactive decay (this comes from the powerful [[nuclear force]]s involved; forces that were still unknown in 1905). In any case, the enormous energy released from radioactive decay (which had been measured by Rutherford) was much more easily measured than the (still small) change in the gross mass of materials, as a result. Einstein's equation, by theory, can give these energies by measuring mass differences before and after reactions, but in practice, these mass differences in 1905 were still too small to be measured in bulk. Prior to this, the ease of measuring radioactive decay energies with a calorimeter was thought possibly likely to allow measurement of changes in mass difference, as a check on Einstein's equation itself. Einstein mentions in his 1905 paper that mass–energy equivalence might perhaps be tested with radioactive decay, which releases enough energy (the quantitative amount known roughly by 1905) to possibly be "weighed," when missing from the system (having been given off as heat). However, radioactivity seemed to proceed at its own unalterable (and quite slow, for radioactives known then) pace, and even when simple nuclear reactions became possible using proton bombardment, the idea that these great amounts of usable energy could be liberated at will with any practicality, proved difficult to substantiate. It had been used as the basis of much speculation, causing Rutherford himself to later reject his ideas of 1904; he was reported in 1933 to have said that: "Anyone who expects a source of power from the transformation of the atom is talking [[moonshine]]."<ref>"We might in these processes obtain very much more energy than the proton supplied, but on the average we could not expect to obtain energy in this way. It was a very poor and inefficient way of producing energy, and anyone who looked for a source of power in the transformation of the atoms was talking moonshine. But the subject was scientifically interesting because it gave insight into the atoms." [http://archive.timesonline.co.uk/ ''The Times'' archives], September 12, 1933, "The British association—breaking down the atom"</ref>
| + | |
| − | | + | |
| − | [[Image:Einstein - Time Magazine - July 1, 1946.jpg|right|thumb|The popular connection between Einstein, {{nowrap|1=''E'' = ''mc''<sup>2</sup>}}, and the [[nuclear weapon|atomic bomb]] was prominently indicated on the cover of ''[[Time (magazine)|Time]]'' magazine in July 1946 by the writing of the equation on the [[mushroom cloud]] itself.]]
| + | |
| − | | + | |
| − | This situation changed dramatically in 1932 with the discovery of the neutron and its mass, allowing mass differences for single [[nuclide]]s and their reactions to be calculated directly, and compared with the sum of masses for the particles that made up their composition. In 1933, the energy released from the reaction of lithium-7 plus protons giving rise to 2 alpha particles (as noted above by Rutherford), allowed Einstein's equation to be tested to an error of ± 0.5%. However, scientists still did not see such reactions as a source of power.
| + | |
| − | | + | |
| − | After the very public demonstration of huge energies released from [[nuclear fission]] after the [[atomic bombings of Hiroshima and Nagasaki]] in 1945, the equation ''E'' = ''mc''<sup>2</sup> became directly linked in the public eye with the power and peril of [[nuclear weapon]]s. The equation was featured as early as page 2 of the [[Smyth Report]], the official 1945 release by the US government on the development of the atomic bomb, and by 1946 the equation was linked closely enough with Einstein's work that the cover of ''[[Time (magazine)|Time]]'' magazine prominently featured a picture of Einstein next to an image of a [[mushroom cloud]] emblazoned with the equation.<ref>[http://www.time.com/time/covers/0,16641,19460701,00.html Cover.] ''Time'' magazine, July 1, 1946.</ref> Einstein himself had only a minor role in the [[Manhattan Project]]: he had [[Einstein–Szilárd letter|cosigned a letter]] to the U.S. President in 1939 urging funding for research into atomic energy, warning that an atomic bomb was theoretically possible. The letter persuaded Roosevelt to devote a significant portion of the wartime budget to atomic research. Without a security clearance, Einstein's only scientific contribution was an analysis of an [[isotope separation]] method in theoretical terms. It was inconsequential, on account of Einstein not being given sufficient information (for security reasons) to fully work on the problem.<ref>Isaacson, ''Einstein: His Life and Universe''.</ref>
| + | |
| − | | + | |
| − | While ''E'' = ''mc''<sup>2</sup> is useful for understanding the amount of energy potentially released in a fission reaction, it was not strictly necessary to develop the weapon, once the fission process was known, and its energy measured at 200 MeV (which was directly possible, using a quantitative Geiger counter, at that time). As the physicist and Manhattan Project participant [[Robert Serber]] put it: "Somehow the popular notion took hold long ago that Einstein's theory of relativity, in particular his famous equation ''E'' = ''mc''<sup>2</sup>, plays some essential role in the theory of fission. Albert Einstein had a part in alerting the United States government to the possibility of building an atomic bomb, but his theory of relativity is not required in discussing fission. The theory of fission is what physicists call a non-relativistic theory, meaning that relativistic effects are too small to affect the dynamics of the fission process significantly."<ref>Robert Serber, ''The Los Alamos Primer: The First Lectures on How to Build an Atomic Bomb'' (University of California Press, 1992), page 7. Note that the quotation is taken from Serber's 1992 version, and is not in the original 1943 [[Los Alamos Primer]] of the same name.</ref> However the association between ''E'' = ''mc''<sup>2</sup> and nuclear energy has since stuck, and because of this association, and its simple expression of the ideas of Albert Einstein himself, it has become "the world's most famous equation".<ref>David Bodanis, ''E = mc<sup>2</sup>: A Biography of the World's Most Famous Equation'' (New York: Walker, 2000).</ref>
| + | |
| − | | + | |
| − | While Serber's view of the strict lack of need to use mass–energy equivalence in designing the atomic bomb is correct, it does not take into account the pivotal role which this relationship played in making the fundamental leap to the initial hypothesis that large atoms were energetically ''allowed'' to split into approximately equal parts (before this energy was in fact measured). In late 1938, while on the winter walk on which they solved the meaning of Hahn's experimental results and introduced the idea that would be called atomic fission, [[Lise Meitner]] and [[Otto Robert Frisch]] made direct use of Einstein's equation to help them understand the quantitative energetics of the reaction which overcame the "surface tension-like" forces holding the nucleus together, and allowed the fission fragments to separate to a configuration from which their charges could force them into an energetic "fission". To do this, they made use of "packing fraction", or nuclear [[binding energy]] values for elements, which Meitner had memorized. These, together with use of ''E'' = ''mc''<sup>2</sup> allowed them to realize on the spot that the basic fission process was energetically possible:
| + | |
| − | | + | |
| − | <blockquote> ...We walked up and down in the snow, I on skis and she on foot. ...and gradually the idea took shape... explained by Bohr's idea that the nucleus is like a liquid drop; such a drop might elongate and divide itself... We knew there were strong forces that would resist, ..just as surface tension. But nuclei differed from ordinary drops. At this point we both sat down on a tree trunk and started to calculate on scraps of paper. ...the Uranium nucleus might indeed be a very wobbly, unstable drop, ready to divide itself... But, ...when the two drops separated they would be driven apart by electrical repulsion, about 200 MeV in all. Fortunately Lise Meitner remembered how to compute the masses of nuclei... and worked out that the two nuclei formed... would be lighter by about one-fifth the mass of a proton. Now whenever mass disappears energy is created, according to Einstein's formula E = mc<sup>2</sup>, and... the mass was just equivalent to 200 MeV; it all fitted!<ref>[http://homepage.mac.com/dtrapp/people/Meitnerium.html A quote from Frisch about the discovery day. Accessed April 4, 2009.]</ref><ref>{{cite book | last=Sime | first=Ruth | title= Lise Meitner: A Life in Physics | publisher = University of California Press | series = California Studies in the History of Science | volume = 13 | year = 1996 | location = Berkeley | pages = 236–237 | isbn = 0-520-20860-9 }}</ref></blockquote>
| + | |
| | | | |
| | ==See also== | | ==See also== |
| − | * [[Binding energy]] (mass defect) | + | *[[Attempts to prove E=mc²]] |
| − | * [[Energy density]] | + | *[[Counterexamples to Relativity]] |
| − | * [[Energy–momentum relation]] | + | *[[Essay:Rebuttal to Counterexamples to Relativity]] |
| − | * [[Inertia]] | + | *[[Logical Flaws in E=mc²]] |
| − | * [[Mass in special relativity]] | + | *[[Essay:Rebuttal to Logical Flaws in E=mc²]] |
| − | * [[Special relativity#Mass, momentum, and energy|Mass, momentum, and energy]] | + | *[[Quantitative Analysis of Alpha Decay]] |
| − | * [[Index of energy articles]]
| + | *[[E^2=(mc^2)^2+(pc)^2]] |
| − | * [[List of wave topics]] | + | |
| − | * [[Outline of energy]]
| + | |
| − | * [[Rotational energy]]
| + | |
| − | | + | |
| − | ==References==
| + | |
| − | {{reflist|colwidth=30em}}
| + | |
| − | {{refbegin}}
| + | |
| − | * {{Citation | author=Bodanis, David | title=E=mc<sup>2</sup>: A Biography of the World's Most Famous Equation | publisher=Berkley Trade | year=2001 | isbn=0-425-18164-2}}
| + | |
| − | * {{Citation | author=Tipler, Paul; Llewellyn, Ralph | title=Modern Physics (4th ed.) | publisher=W. H. Freeman | year=2002 | isbn=0-7167-4345-0}}
| + | |
| − | * {{Citation | first=Ronald C. | last=Lasky | coauthors= | title=What is the significance of E = mc<sup>2</sup>? And what does it mean? | date=April 23, 2007 | publisher= [[Scientific American]]| url =http://www.sciam.com/article.cfm?id=significance-e-mc-2-means | work =Scientific American | pages = | accessdate = | language = }}
| + | |
| − | {{refend}}
| + | |
| − | | + | |
| − | ==External links==
| + | |
| − | {{Wikisourcepar|Relativity: The Special and General Theory}}
| + | |
| − | {{Commons category|Einstein formula}}
| + | |
| − | * [http://plato.stanford.edu/entries/equivME The Equivalence of Mass and Energy] – Entry in the Stanford Encyclopedia of Philosophy
| + | |
| − | * [http://relativity.livingreviews.org/ Living Reviews in Relativity] – An open access, peer-referred, solely online physics journal publishing invited reviews covering all areas of relativity research.
| + | |
| − | *[http://fotonowy.pl/index.php?main_page=page&id=6 A shortcut to ''E''=''mc''<sup>2</sup>] – An easy to understand, high-school level derivation of the ''E''=''mc''<sup>2</sup> formula.
| + | |
| − | * [http://www.mathpages.com/home/kmath600/kmath600.htm Einstein on the Inertia of Energy] – MathPages
| + | |
| − | | + | |
| − | {{Einstein}}
| + | |
| − | | + | |
| − | {{DEFAULTSORT:Mass–Energy Equivalence}}
| + | |
| − | [[Category:Mass]]
| + | |
| − | [[Category:Energy (physics)]]
| + | |
| − | [[Category:Special relativity]]
| + | |
| − | [[Category:Equations]]
| + | |
| − | [[Category:Albert Einstein]]
| + | |
| − | [[Category:1905 introductions]]
| + | |
| | | | |
| − | {{Link FA|eu}}
| + | == References == |
| − | {{Link GA|ru}}
| + | <references /> |
| − | [[ar:تكافؤ المادة والطاقة]]
| + | [[Category:Relativity]] |
| − | [[az:Kütlə və enerjinin ekvivalentliyi]]
| + | [[Category:Laws of Physics]] |
| − | [[bg:Равенство на маса и енергия]]
| + | [[Category:Physics]] |
| − | [[br:E=mc²]]
| + | |
| − | [[ca:Equivalència entre massa i energia]]
| + | |
| − | [[cs:E=mc²]]
| + | |
| − | [[da:E=mc²]]
| + | |
| − | [[de:Äquivalenz von Masse und Energie]]
| + | |
| − | [[et:E=mc²]]
| + | |
| − | [[el:Ισοδυναμία μάζας-ενέργειας]]
| + | |
| − | [[es:Equivalencia entre masa y energía]] | + | |
| − | [[eu:E=mc²]] | + | |
| − | [[fa:همارزی جرم و انرژی]]
| + | |
| − | [[fr:E=mc2]]
| + | |
| − | [[fy:Massa-enerzjyrelaasje]]
| + | |
| − | [[gl:E=mc²]]
| + | |
| − | [[ko:질량-에너지 동등성]]
| + | |
| − | [[hy:Զանգված-էներգիա համարժեքություն]]
| + | |
| − | [[hr:Ekvivalencija mase i energije]]
| + | |
| − | [[id:E=mc²]]
| + | |
| − | [[it:E=mc²]]
| + | |
| − | [[he:E=mc²]]
| + | |
| − | [[jv:E=mc²]]
| + | |
| − | [[lad:E=mc²]]
| + | |
| − | [[la:Aequatio massae et energiae]]
| + | |
| − | [[hu:Tömeg–energia ekvivalencia]]
| + | |
| − | [[mk:Еднаквост на масата и енергијата]]
| + | |
| − | [[ml:E = mc²]]
| + | |
| − | [[ms:E=mc²]]
| + | |
| − | [[nl:Massa-energierelatie]]
| + | |
| − | [[ja:E=mc²]]
| + | |
| − | [[nap:E=mc²]]
| + | |
| − | [[no:Masseenergiloven]]
| + | |
| − | [[nn:Masseenergilova]]
| + | |
| − | [[km:រូបមន្តសមមូលម៉ាស់-ថាមពល]]
| + | |
| − | [[pl:Równoważność masy i energii]]
| + | |
| − | [[pt:Equivalência massa-energia]]
| + | |
| − | [[ro:Echivalență masă–energie]]
| + | |
| − | [[ru:Эквивалентность массы и энергии]]
| + | |
| − | [[scn:E=mc²]]
| + | |
| − | [[si:ස්කන්ධ–ශක්ති තුල්යතාවය]]
| + | |
| − | [[sk:Einsteinov vzťah]]
| + | |
| − | [[sl:E = mc²]]
| + | |
| − | [[sr:E=mc²]]
| + | |
| − | [[sh:Ekvivalencija mase i energije]]
| + | |
| − | [[su:E=mc²]]
| + | |
| − | [[fi:E=mc²]]
| + | |
| − | [[sv:E = mc²]]
| + | |
| − | [[tl:Pagkakatumbas na masa-enerhiya]]
| + | |
| − | [[ta:திணிவு-ஆற்றல் சமன்பாடு]]
| + | |
| − | [[th:ความสมมูลระหว่างมวล-พลังงาน]]
| + | |
| − | [[tr:E=mc²]]
| + | |
| − | [[uk:Формула Ейнштейна]]
| + | |
| − | [[vi:Sự tương đương khối lượng-năng lượng]]
| + | |
| − | [[yi:E=mc²]]
| + | |
| − | [[zh:質能等價]] | + | |
The formula asserts that the mass of an object, at constant energy, magically varies precisely in inverse proportion to the square of a change in the speed of light over time,[4] which violates conservation of mass and disagrees with commonsense.[5]
It has been known for a long time that radiation has a mass equivalence, which was correctly derived by Henri Poincaré in 1904,[14] but the equation E=mc² makes a claim far beyond that limited circumstance:
The equation is extremely famous, and just as extremely misunderstood, in popular culture. Among the more outlandish claims are statements to the effect that "E=mc² holds the secret of the atomic bomb."[15]
The equation has acquired something of a "cult" status. In the USA, the popular Twilight Zone series featured E=mc² prominently, giving the equation greater currency with the public. The song Einstein A Go-Go by the band Landscape had a similar effect in the UK in the 1980s. The equation was the title of a single by Big Audio Dynamite in 1985, and an album by Mariah Carey in 2008. Some movies have been themed on this equation.[17] The equation, along with a picture of a mushroom cloud and a picture of Albert Einstein, were featured on the front cover of an issue of Time magazine in 1946. All of this is disappointing when one considers how few people actually understand what the equation is saying.
A number of science writers—both serious scientists and science popularizers—have at various times written their own explanation of the equation. Some of these are helpful; many are not. One of the better ones, though not without its share of nonsense, is a NOVA series by the Public Broadcasting Service[18]
In 2005 The PBS NOVA series also asked 10 physicists to describe the equation in layman's terms.[19] Here is a sample of five of the statements:
Of these, only the Sheldon Glashow quote makes a specific and meaningful statement about what the equation means.
The equation is about energy, both kinetic energy and potential energy.
Kinetic energy is actual visible energy, that is, energy of things that are in motion. It's the energy of a thrown baseball. Radiation (for example, light) also counts as kinetic energy—it's the motion of photons. Light carries energy, force, and momentum. The force carried by light is not as obvious as the force of a thrown baseball, but it is there. The force of sunlight has been proposed for long-term space travel. It is also the force that causes the Pioneer anomaly and the force that makes a comet's tail stream away from the Sun.
Potential energy is the other kind—"hidden" energy. It can become kinetic energy, or vice-versa. A wound up spring, a charged battery, a stretched rubber band, a mixture of gasoline and air, an explosive, and a radioactive atom, all have potential energy. It's what is needed to make the principle of conservation of energy work. That is, when kinetic energy comes into existence, it's because potential energy was converted into kinetic energy. The wound-up spring of a clock has potential energy, that runs down over time, being converted into the kinetic energy of the ticking sound. A battery has potential energy that runs down when it provides electricity to make things move. Various chemical substances have characteristic amounts of potential energy, that may be converted to or from kinetic energy when chemical reactions occur. For example, Sodium and Chlorine have more potential energy than Sodium Chloride. Explosives have more potential energy than their constituent atoms. Radioactive atoms have more potential energy than their "daughter" atoms.
Hundreds of years of research by chemists (and, before that, the alchemists) worked out the potential energies that are characteristic of various substances, and that the potential and kinetic energies are accurately converted from one to the other, leading to the principle of conservation of total energy.
That is, it weighs something. Whenever anything has potential energy of any kind in it, improbable as this may sound, it weighs more. The proportionality constant is 1/c2, or 1.11 x 10−17 kilograms per joule. A fresh battery weighs more than a spent one, a wound-up alarm clock weighs more than a run-down one, etc.
Now that's way too small to measure for anything other than nuclear reactions, which is why it escaped everyone's notice for so long. But it has been measured and experimentally verified for nuclear transformations all across the periodic table.
The nonzero mass of potential energy, and the equation E=mc², were determined on theoretical grounds, before any experimental observations were made. The logic of this follows from these assumptions:
Keep in mind that, under special relativity, it's not just space and time that need to be redefined. The definitions of momentum and energy need to change also. This is necessary so that the conservation of energy and of momentum will be absolutely precise in all circumstances.
Under classical Newtonian mechanics, the momentum and kinetic energy of a moving mass are
respectively. But under special relativity they are
One can verify that, in the non-relativistic limit, the relativistic values converge to the classical ones.
Measuring the effect requires process that release vastly more energy than ordinary chemical processes. The discovery of Radium and Polonium around 1898 gave a tantalizing hint that there were processes that released far more energy than chemical processes could account for. These elements continuously released measurable heat, and also glowed in the dark.
It would take more than a decade to develop an understanding of the nuclear process involved. The first thing that was required was accurate knowledge of atomic weights.
Atomic weights of the various elements were first measured, with accuracy of a few decimal places, by J. J. Berzelius in the late 1820s. This required extremely painstaking (for the time) measurements. The figures were refined to even more accuracy by J. A. R. Newlands in the 1860s. The values were accurate enough to clearly show the rather interesting property that the atomic weights were nearly integers, but not exactly so. The reason for this would turn out to be partly because of different isotopes (discovered by Frederick Soddy in 1913) and partly because of E=mc2.
In 1907 Rutherford determined that the "alpha" radiation from Radium was Helium. In 1911 he formulated the theory of the nucleus. In 1919 he demonstrated that nuclear transmutations could take place, such as
The discovery of Radon, and much further investigation, revealed that the behavior of Radium was
Accurate ways of measuring speed of a charged particle, by deflecting it in a magnetic field, had been developed by then, so that, by very painstaking observation and measurement, it was determined that the first alpha particle (Helium nucleus) had an energy of 4.78 MeV and the second an energy of 5.49 Mev. This confirmed E=mc2 up to the accuracy of the measurements. The equation, along with knowledge of isotope mixes, now explained why the atomic weights appearing in the periodic table were nearly integers, but not exactly so.
Around 1925, the development of the mass spectrograph, by Francis Aston, made it possible to measure atomic weights to extreme precision.
The 1932 Cockcroft-Walton experiment, described in more detail below, started to make the equation famous by confirming it, with reasonable accuracy, for an artificially induced nuclear reaction. (Confirming E=mc2 was not a goal of the experiment; it was an incidental consequence. The equation had already been known and understood for many years.)
In the decades since, nuclear transmutations have been performed, in particle accelerators, all over the periodic table, observing in detail the properties of various isotopes. These have confirmed E=mc2 with great precision. See Quantitative Analysis of Alpha Decay.
This experiment is not one usually cited as validating E=mc². That was not its goal. The generally accepted important tests of this equation are the measurements of alpha decay energies, described above.
In 1932 English physicist John Cockcroft and Irish physicist Ernest Walton performed the first artificial nuclear transmutation of nuclei, for which they were awarded the 1951 Nobel Prize in physics.[23] The award was for "their pioneer work on the transmutation of atomic nuclei by artificially accelerated atomic particles."[24]
Verifying E=mc² was not the goal of the experiment, and the Nobel prize was awarded for the transmutation itself, not any verification of the equation. This experiment could not have proved any general truth to the equation, since it was a test of just one specific reaction. But data from this experiment was consistent with the equation for the particular transmutation involved.
Accurate measurements and detailed calculations allowed for verifying the theoretical values with an accuracy of ±0.5%. This was the first time a nucleus was artificially split, and thereby the first transmutation of elements using accelerated particles:
For most types of physical interactions, the masses of the initial reactants and of the final products match so closely that it is essentially impossible to measure any difference. But for nuclear reactions, the difference is measurable. That difference is related to the energy absorbed or released, described by the equation E=mc². (The equation applies to all interactions; the fact that nuclear interactions are the only ones for which the mass difference is measurable has led people to believe, wrongly, that E=mc² applies only to nuclear interactions.)
Nuclear fission, which is the basis for nuclear energy, was discovered in experiments by Otto Hahn and Fritz Strassman, and analyzed by Lise Meitner, in 1938.
The mass of the Uranium atom is 235.04393, and the sum of the masses of the products is 234.866503. The difference is .177427 amu, or, using the E=mc² equation, 165 million electron volts. (The generally accepted value for the total energy released by Uranium fission, including secondary decays, is about 200 million electron volts.)
The insight that the conversion from Uranium to Barium was caused by complete fission of the atom was made by Lise Meitner in December, 1938. She had the approximate "mass defect" quantities memorized, and so she worked out in her head, using the E=mc² equation, that there would be this enormous release of energy. This release was observed shortly thereafter, and the result is nuclear power and nuclear weapons.
Here is another example of the use of this formula in physics calculations. In 2011 there were reports that high-energy neutrinos had been observed traveling at a speed faster than the speed of light in an experiment at the Gran Sasso laboratory in Italy. Specifically, they seemed to have arrived at the detector 60 nanoseconds faster than light would have. Relativity doesn't allow that, and, since neutrinos have nonzero (but incredibly tiny) mass, they aren't even supposed to travel at the speed of light.
While the equation was historically developed on theoretical grounds as an inevitable consequence of special relativity, it is possible to deduce it purely from empirical observation.
So, for the purposes of this section, imagine that one is in the era of "classical physics"; prior to 1900 or so. Relativity has not been invented, but, inexplicably, nuclear physics has. Imagine that the phenomena of radioactivity and nuclear fission have been observed, without any knowledge of relativity.
A well-accepted physical law of classical physics was the law of conservation of mass. This was not easy to deduce. It required careful analysis of such phenomena as combustion, in the 1700s, to eliminate the various confounding sub-phenomena that made the law difficult to see. But, by 1900, the law was well established:
For example, the mass of a TNT molecule is 227.1311 Daltons, or 227.1311 g/mol, which is, for all practical purposes, the same as the mass of its constituent Carbon, Hydrogen, Nitrogen, and Oxygen atoms. It is essentially impossible to measure the difference. The principle of conservation of mass is upheld.
But when nuclear phenomena are discovered, we notice something different. The masses of the result particles after an event (e.g. alpha decay, nuclear fission, or artificial transmutation) is measurably less than the masses of the original particle(s). With the invention of the mass spectrometer around 1920, it became possible to measure atomic weights of various isotopes with great precision.
Radium-226 decays into Radon-222 by emission of an alpha particle with an energy of 4.78 MeV.
That number of Radon-222 atoms has mass .98226836 kg. That number of alpha particles has mass .01770863 kg.
The mass lost is .00002301 kg.
That number of Polonium-218 atoms has mass .98194467 kg. That number of alpha particles has mass .01802830 kg.
The mass lost is .00002703 kg.
Each emitted alpha particle has energy of 5.49 MeV. The total alpha energy from the decay of 1 kg of polonium is 2.39 * 1012 Joules.
Making special cases like this is unsatisfactory, of course.
We do this for a few other interactions, including the explosion of TNT. This would include the Lithium-plus-Hydrogen and Uranium fission phenomena described above. We won't bother with the details. As observational scientists, we look for patterns in the behavior of nature. We make a table:
What we thought was exact conservation is just very nearly exact, and we hadn't been able to measure it before.
But maybe there's more. This constant has dimensions of kilograms per Joule. From high-school physics, we know that that is seconds squared divided by meters squared. That is, it is the reciprocal of the square of a velocity. We calculate that velocity. It is about 2.97 * 108 meters per second. Very close to the speed of light! Very interesting! (The calculations above were not extremely precise. The formula has been verified with great precision, but not here.)
We don't understand why (that will have to wait for the invention of relativity), but we can formulate a hypothesis:
We don't have to give the units any more, since everything is now dimensionally correct.

 , which 1.11 x 10−17 kilograms per joule), it is essentially impossible to check this equation for normal processes. For example, a flashlight battery loses about 1 picogram of mass when it discharges, and the resultant atoms from the detonation of 1 kilogram of TNT weigh 47 nanograms less than the TNT. Even if all the particles of smoke and gas could be collected reliably, the difference couldn't be detected.
, which 1.11 x 10−17 kilograms per joule), it is essentially impossible to check this equation for normal processes. For example, a flashlight battery loses about 1 picogram of mass when it discharges, and the resultant atoms from the detonation of 1 kilogram of TNT weigh 47 nanograms less than the TNT. Even if all the particles of smoke and gas could be collected reliably, the difference couldn't be detected.
 , one would get v=110x1012 meters per second. This is about 370,000 times the speed of light, something that scientists would certainly have noticed. In fact, with special relativity, the speed is just under the speed of light, such that the neutrinos should be received at the detector about .26x10−24 seconds (.26 yoctoseconds) later than the speed of light itself. This is far too small to measure—15 orders of magnitude smaller than the resolution of the GPS signals in the experiment.
, one would get v=110x1012 meters per second. This is about 370,000 times the speed of light, something that scientists would certainly have noticed. In fact, with special relativity, the speed is just under the speed of light, such that the neutrinos should be received at the detector about .26x10−24 seconds (.26 yoctoseconds) later than the speed of light itself. This is far too small to measure—15 orders of magnitude smaller than the resolution of the GPS signals in the experiment.
 atoms. (The numerator is Avogadro's number, and the denominator is the atomic weight of Radium-226.) This is 2.6643647 * 1024 atoms.
atoms. (The numerator is Avogadro's number, and the denominator is the atomic weight of Radium-226.) This is 2.6643647 * 1024 atoms.
 atoms. This is 2.7124611 * 1024 atoms.
atoms. This is 2.7124611 * 1024 atoms.
 times the amount of energy released.
times the amount of energy released. and
and  appearing in his equations. Specifically,
appearing in his equations. Specifically,  has the dimensions of seconds squared divided by meters squared, and that:
has the dimensions of seconds squared divided by meters squared, and that: